Isoprenoids are a huge group of compounds that include primary metabolites such as carotenoids, chlorophylls, and hormones, as well as a plethora of specialized secondary metabolites. In addition to their importance in the physiology of plants (and of other kingdoms of life), isoprenoids have drawn attention in recent years for their applications in human health (reviewed in Kirby and Keasling, 2009). Isoprenoids are produced from two common 5-carbon isomers, isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP; reviewed in Vranová et al., 2013) that serve as the building blocks for isoprenoids. The polyisoprenoids dolichol and polyprenol comprise a class of long, linear isoprenoids greater of 45 carbons or greater (see Figure). Whereas dolichols are well known for being essential in N-glycosylation, the functions of plastid-localized polyprenols are less clear (reviewed in Surmacz and Swiezewska, 2011). New work from Akhtar et al. (2017) exploring the biosynthesis of polyprenols in plants indicates that they could be important in thylakoid membrane dynamics.
A two-component complex required for dolichol synthesis (Brasher et al. 2015) in plants includes a cis-prenyltranferase (CPT), prompting Akhtar and coworkers to search among the nine Arabidopsis thaliana CPTs for those expressed in green tissue—where polyprenols are known to accumulate—and phylogenetically distinct from the CPT that produces dolichol. These criteria led the authors to CPT7 as a candidate for polyprenol biosynthesis.
Akhtar et al. found that wild-type leaves contained medium-chain polyprenols, composed of 9–11 isoprenoid units (45–55 carbons), mostly as free alcohols. By contrast, a homozygous cpt7 T-DNA mutant was missing these classes of polyprenols and CPT7 RNAi lines were decreased for them, whereas CPT7 overexpressors had markedly increased or at least wild-type levels. Further, recombinant CPT7 was active in adding IPP units to all three tested intermediate substrates, with a strong preference for farnesyl diphosphate (FPP) and geranylgeranyl diphosphate (GGPP).
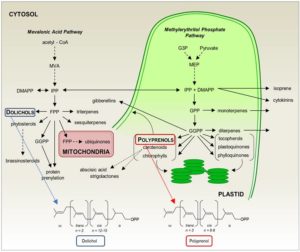 As FPP and GGDP are produced in different locations within the cell (see Figure), Akhtar and coauthors characterized the subcellular localization of CPT7. A combination of immunodetection and in vivo localization of fluorescent protein fusions revealed CPT7 in the stromal fraction, where most of the medium-chain CPT activity in the chloroplast resides. Thus, it appears that CPT7 functions as the primary enzyme for stromal polyprenol biosynthesis.
As FPP and GGDP are produced in different locations within the cell (see Figure), Akhtar and coauthors characterized the subcellular localization of CPT7. A combination of immunodetection and in vivo localization of fluorescent protein fusions revealed CPT7 in the stromal fraction, where most of the medium-chain CPT activity in the chloroplast resides. Thus, it appears that CPT7 functions as the primary enzyme for stromal polyprenol biosynthesis.
However, polyprenols are quite hydrophobic, and Akhtar et al. reasoned that they are unlikely to accumulate in the aqueous environment of chloroplast stroma, despite the stromal localization of CPT7. Indeed, polyprenols were present mainly in the thylakoid membrane fraction of wild type chloroplasts. Consistent with this localization, the CPT7 RNAi and CPT7 overexpression lines had lower and higher levels, respectively, of medium-chain polyprenols in the thylakoid fractions.
Akhtar et al. took advantage of these lines to address the possible roles of polyprenols in the thylakoid membrane. In artificial membranes, polyprenols are reported to increase membrane fluidity. In the protein-dense thylakoid membranes, however, fluorescence anisotropy analysis revealed that the lines with less polyprenol had greater membrane fluidity. These lines also had lower photosystem II operating efficiency, due to a lower rate of electron transport. These analyses thus suggest that medium-chain polyprenols—produced by CPT7—help determine the biophysical characteristics of thylakoid membranes by restricting fluidity, with important consequences to photosynthetic performance.
REFERENCES
Akhtar, T.A., Surowiecki, P., Siekierska, H., Kania, M., Van Gelder, K., Rea, K., Virta, L., Maritza Vatta, Gawarecka, K., Wojcik, J., Danikiewicz, W., Buszewicz, D., Swiezewska, E., Surmacz, L. (2017). Polyprenols are Synthesized by a Plastidial cis-Prenyltransferase and Influence Photosynthetic Performance. Plant Cell. doi: 10.1105/tpc.16.00796.
Brasher, M.I., Surmacz, L., Leong, B., Pitcher, J., Swiezewska, E., Pichersky, E., Akhtar, T.A. (2015) A two-component enzyme complex is required for dolichol biosynthesis in tomato. Plant J. 82: 903-914.
Kirby, J., and Keasling, J.D. (2009) Biosynthesis of plant isoprenoids: perspectives for microbial engineering. Annu. Rev. Plant. Biol. 60: 335-355.
Surmacz, L., and Swiezewska, E. (2011) Polyisoprenoids – Secondary metabolites or physiologically important superlipids? Biochem. Biophys. Res. Commun. 407: 627-632.
Vranová, E., Coman, D., and Gruissem, W. (2013) Network analysis of the MVA and MEP pathways for isoprenoid synthesis. Annu. Rev. Plant Biol. 64: 665-700.
 Key points:
Key points:

 This week’s post was written by Jonathan Ingram, Senior Commissioning Editor / Science Writer for the
This week’s post was written by Jonathan Ingram, Senior Commissioning Editor / Science Writer for the 
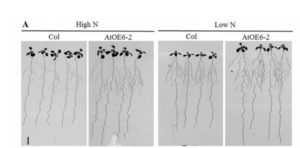 In plants, there are two biosynthetic pathways for the production of the plant hormone indole-3-acetic acid (IAA), namely the Trp-dependent and the Trp-independent pathways. Shao et al. (
In plants, there are two biosynthetic pathways for the production of the plant hormone indole-3-acetic acid (IAA), namely the Trp-dependent and the Trp-independent pathways. Shao et al. ( Legume roots form two types of organs, lateral roots and symbiotic nodules, which participate, respectively, in the uptake of water and mineral nutrients and in nitrogen fixation. Since both organs have considerable impacts on plant growth, understanding the mechanisms underlying the development of lateral roots and nodules is crucial to improve agronomical traits in legumes. MicroRNA390 (miR390) is an evolutionarily conserved miRNA that targets non-coding Trans Acting Short Interference RNA3 (TAS3). Cleavage of TAS3 by ARGONAUTE7 results in the production of tasiRNAs, which target mRNAs encoding AUXIN RESPONSE FACTOR 2 (ARF2), ARF3 and ARF4. The miR390/TAS3 pathway plays key roles in plant development. tasiARFs suppress the juvenile to adult phase transition in Arabidopsis (Arbidopsis thaliana) and are required for leaf patterning and leaf polarity in different plant species, including the two model leguminous plants L. japonicus and M. truncatula. The miR390/TAS3 pathway also defines a network that quantitatively controls lateral root growth in Arabidopsis.
Legume roots form two types of organs, lateral roots and symbiotic nodules, which participate, respectively, in the uptake of water and mineral nutrients and in nitrogen fixation. Since both organs have considerable impacts on plant growth, understanding the mechanisms underlying the development of lateral roots and nodules is crucial to improve agronomical traits in legumes. MicroRNA390 (miR390) is an evolutionarily conserved miRNA that targets non-coding Trans Acting Short Interference RNA3 (TAS3). Cleavage of TAS3 by ARGONAUTE7 results in the production of tasiRNAs, which target mRNAs encoding AUXIN RESPONSE FACTOR 2 (ARF2), ARF3 and ARF4. The miR390/TAS3 pathway plays key roles in plant development. tasiARFs suppress the juvenile to adult phase transition in Arabidopsis (Arbidopsis thaliana) and are required for leaf patterning and leaf polarity in different plant species, including the two model leguminous plants L. japonicus and M. truncatula. The miR390/TAS3 pathway also defines a network that quantitatively controls lateral root growth in Arabidopsis. 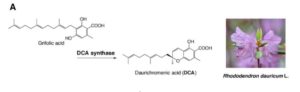 Rhododendron dauricum (Ericaceae), a native of northeastern Asia, produces unique secondary metabolites including daurichromenic acid (DCA). DCA has attracted considerable attention as a medicinal resource because this compound is one of the most effective natural products with anti-HIV properties in cell culture. Thus, chemical synthesis of DCA has been extensively studied over the past few years. Previously, a partial characterization has been made of an oxidocyclase, named DCA synthase, using a crude protein extract from young leaves of R. dauricum. DCA synthase is an enzyme that catalyzes the stereoselective oxidative cyclization of farnesyl moiety of grifolic acid to form DCA. Unlike P-450 type cyclases involved in glyceollin and furanocoumarin biosynthesis, DCA synthase is a soluble protein, and does not need exogenously added cofactors for the reaction. Remarkably, these features are similar to those reported for cannabinoid synthases from Cannabis sativa. Iijima et al. (
Rhododendron dauricum (Ericaceae), a native of northeastern Asia, produces unique secondary metabolites including daurichromenic acid (DCA). DCA has attracted considerable attention as a medicinal resource because this compound is one of the most effective natural products with anti-HIV properties in cell culture. Thus, chemical synthesis of DCA has been extensively studied over the past few years. Previously, a partial characterization has been made of an oxidocyclase, named DCA synthase, using a crude protein extract from young leaves of R. dauricum. DCA synthase is an enzyme that catalyzes the stereoselective oxidative cyclization of farnesyl moiety of grifolic acid to form DCA. Unlike P-450 type cyclases involved in glyceollin and furanocoumarin biosynthesis, DCA synthase is a soluble protein, and does not need exogenously added cofactors for the reaction. Remarkably, these features are similar to those reported for cannabinoid synthases from Cannabis sativa. Iijima et al. ( Prompted by this finding,
Prompted by this finding,  As FPP and GGDP are produced in different locations within the cell (see Figure), Akhtar and coauthors characterized the subcellular localization of CPT7. A combination of immunodetection and in vivo localization of fluorescent protein fusions revealed CPT7 in the stromal fraction, where most of the medium-chain CPT activity in the chloroplast resides. Thus, it appears that CPT7 functions as the primary enzyme for stromal polyprenol biosynthesis.
As FPP and GGDP are produced in different locations within the cell (see Figure), Akhtar and coauthors characterized the subcellular localization of CPT7. A combination of immunodetection and in vivo localization of fluorescent protein fusions revealed CPT7 in the stromal fraction, where most of the medium-chain CPT activity in the chloroplast resides. Thus, it appears that CPT7 functions as the primary enzyme for stromal polyprenol biosynthesis. This week’s edition of What We’re Reading is guest edited by Dr.
This week’s edition of What We’re Reading is guest edited by Dr.  Gene expression patterns are often used to assign function, but how tight is the correlation between expression and function? Zhang et al. addressed this question by investigating whether orthologous genes in two species (including the two subgenomes of maize) show similar patterns of expression. Specifically, they carried out a genome-wide transcriptional comparison of cold-response in sorghum and maize. They found that most orthologous genes were not similarly regulated in maize and sorghum, although early-expressed cold-response genes showed greater cross-species conservation than later genes. Further, genes with conserved transcriptional regulation patterns had a low ratio of non-synonymous to synonymous substitutions, suggesting stronger selection. The authors suggest that cross-species expression comparisons can reveal functionally important genes. Plant Cell.
Gene expression patterns are often used to assign function, but how tight is the correlation between expression and function? Zhang et al. addressed this question by investigating whether orthologous genes in two species (including the two subgenomes of maize) show similar patterns of expression. Specifically, they carried out a genome-wide transcriptional comparison of cold-response in sorghum and maize. They found that most orthologous genes were not similarly regulated in maize and sorghum, although early-expressed cold-response genes showed greater cross-species conservation than later genes. Further, genes with conserved transcriptional regulation patterns had a low ratio of non-synonymous to synonymous substitutions, suggesting stronger selection. The authors suggest that cross-species expression comparisons can reveal functionally important genes. Plant Cell.  By measuring the length of Arabidopsis and rice roots treated with synthetic peptides, Pruitt et al. showed that RaxX, a peptide produced by the rice pathogen Xanthomonas oryzae pv. oryzae (Xoo), mimicked the growth-stimulating activity of plant peptides containing sulfated tyrosine residues (PSYs). They propose that the pathogen produces RaxX to promote growth of infected tissues, and they also show that RaxX-deficient Xanthomonas have decreased virulence. Unlike growth-promoting PSYs, the pathogen-derived peptide induces immune responses, indicating that the plant is able to distinguish between the endogenous and pathogen-derived peptides. New Phytol.
By measuring the length of Arabidopsis and rice roots treated with synthetic peptides, Pruitt et al. showed that RaxX, a peptide produced by the rice pathogen Xanthomonas oryzae pv. oryzae (Xoo), mimicked the growth-stimulating activity of plant peptides containing sulfated tyrosine residues (PSYs). They propose that the pathogen produces RaxX to promote growth of infected tissues, and they also show that RaxX-deficient Xanthomonas have decreased virulence. Unlike growth-promoting PSYs, the pathogen-derived peptide induces immune responses, indicating that the plant is able to distinguish between the endogenous and pathogen-derived peptides. New Phytol.  Ribeiro et al. describe functional aspects of the rice gene OsAPX4 encoding a peroxisomal ascorbate peroxidase, which shows the highest level of expression in leaves. RNAi silencing of this gene did not lead to an increase in the intracellular H2O2 levels and no change was observed in growing plants. However, a drastic change was seen in aging plants which exhibited early senescence. The study suggests that OsAPX4 plays a significant role in ROS signalling during leaf senescence Plant Science.
Ribeiro et al. describe functional aspects of the rice gene OsAPX4 encoding a peroxisomal ascorbate peroxidase, which shows the highest level of expression in leaves. RNAi silencing of this gene did not lead to an increase in the intracellular H2O2 levels and no change was observed in growing plants. However, a drastic change was seen in aging plants which exhibited early senescence. The study suggests that OsAPX4 plays a significant role in ROS signalling during leaf senescence Plant Science.  Mishra et al. (2017) characterized two GPATs (glycerol-3-phosphate acyltransferase) JcGPAT1 and JcGPAT2 in the biofuel seed oil crop Jatropha curcas. These GPATs showed sequence homology with Arabidopsis acyltransferase-1 (ATS1) and Arabidopsis GPAT (AtGPAT9) respectively. Subcellular localization studies indicated that JcGPAT1 is localized in plastids whereas JcGPAT2 is localized in the endoplasmic reticulum. Overexpression of these enzymes in Arabidopsis thaliana led to an increase in total seed oil content – as much as a 43-60% increase in transgenic lines overexpressing JcGPAT2. This study highlights the role of these GPATs in oil biosynthesis in Jatropha curcas. Plant Science
Mishra et al. (2017) characterized two GPATs (glycerol-3-phosphate acyltransferase) JcGPAT1 and JcGPAT2 in the biofuel seed oil crop Jatropha curcas. These GPATs showed sequence homology with Arabidopsis acyltransferase-1 (ATS1) and Arabidopsis GPAT (AtGPAT9) respectively. Subcellular localization studies indicated that JcGPAT1 is localized in plastids whereas JcGPAT2 is localized in the endoplasmic reticulum. Overexpression of these enzymes in Arabidopsis thaliana led to an increase in total seed oil content – as much as a 43-60% increase in transgenic lines overexpressing JcGPAT2. This study highlights the role of these GPATs in oil biosynthesis in Jatropha curcas. Plant Science  The stringent response in bacteria is a global transcriptional response to stress, mediated by the second messenger ppGpp (guanosine 5′-diphosphate 3′-diphosphate) which is synthesized by the action of guanosine 5′-triphosphate 3′-diphosphate pyrophosphatase (GppA) or exopolyphosphatase (Ppx). Recently it was shown that plants also exhibit a stringent response mediated by ppGpp, but the origin of this response in plants has not been resolved. Ito et al. investigated the distribution of the gppA/ppx gene families as well as the RSH hydrolase gene family. Their data indicate that genes involved in the stringent response were likely introduced to plants from various bacterial phyla by lateral gene transfer events. Moreover, the results suggest that plant RSH homologs likely function in the plastid, whereas the plant gppA/ppx homologs may function in the cytosol. J. Plant Research
The stringent response in bacteria is a global transcriptional response to stress, mediated by the second messenger ppGpp (guanosine 5′-diphosphate 3′-diphosphate) which is synthesized by the action of guanosine 5′-triphosphate 3′-diphosphate pyrophosphatase (GppA) or exopolyphosphatase (Ppx). Recently it was shown that plants also exhibit a stringent response mediated by ppGpp, but the origin of this response in plants has not been resolved. Ito et al. investigated the distribution of the gppA/ppx gene families as well as the RSH hydrolase gene family. Their data indicate that genes involved in the stringent response were likely introduced to plants from various bacterial phyla by lateral gene transfer events. Moreover, the results suggest that plant RSH homologs likely function in the plastid, whereas the plant gppA/ppx homologs may function in the cytosol. J. Plant Research  Dehydrins are proteins that have important roles in dehydration stress in plants. Abedini et al. carried out a phylogenetic analysis of barley dehydrins and investigated physiological and gene expression levels in tolerant (HV1), and susceptible (HV2) cultivars and a drought tolerant Iranian wild barley genotype (Spontaneum; HS) subjected to water stress and after recovery. They found notable differences in physiological responses and dehydrin expression levels in the drought tolerant varieties as compared to the susceptible variety, as well as between the wild and cultivated genotypes, supporting the role for dehydrins in vegetative drought tolerance. As barley is an important grain in arid regions, the study will contribute to future breeding efforts for drought tolerance J. Plant Research
Dehydrins are proteins that have important roles in dehydration stress in plants. Abedini et al. carried out a phylogenetic analysis of barley dehydrins and investigated physiological and gene expression levels in tolerant (HV1), and susceptible (HV2) cultivars and a drought tolerant Iranian wild barley genotype (Spontaneum; HS) subjected to water stress and after recovery. They found notable differences in physiological responses and dehydrin expression levels in the drought tolerant varieties as compared to the susceptible variety, as well as between the wild and cultivated genotypes, supporting the role for dehydrins in vegetative drought tolerance. As barley is an important grain in arid regions, the study will contribute to future breeding efforts for drought tolerance J. Plant Research  Peroxygenases catalyze the hydroperoxide dependent epoxidation of unsaturated fatty acids. Benaragama et al. identified two novel peroxygenases (AsPXG2 and AsPXG3) in oat (Avena sativa L.) AsPXG2 and AsPXG3 share structural similarity by having a single transmembrane domain, conserved histidines for heme-binding as well as a conserved EF-hand motif. However, they show only 50% amino acid identity with each other, and the activity of heterologously-expressed AsPXG3 is much higher than that of AsPXG2. Site-directed mutagenesis of AsPXG3 showed that replacement of two conserved histidines by alanine resulted in complete loss of activity, and substitution of three conserved residues surrounding the two histidines resulted in reduction of enzymatic activity by more than 80%, suggesting that these conserved residues are located near the catalytic sites, where they coordinate the heme group and define the shape and size of the catalytic site. Planta
Peroxygenases catalyze the hydroperoxide dependent epoxidation of unsaturated fatty acids. Benaragama et al. identified two novel peroxygenases (AsPXG2 and AsPXG3) in oat (Avena sativa L.) AsPXG2 and AsPXG3 share structural similarity by having a single transmembrane domain, conserved histidines for heme-binding as well as a conserved EF-hand motif. However, they show only 50% amino acid identity with each other, and the activity of heterologously-expressed AsPXG3 is much higher than that of AsPXG2. Site-directed mutagenesis of AsPXG3 showed that replacement of two conserved histidines by alanine resulted in complete loss of activity, and substitution of three conserved residues surrounding the two histidines resulted in reduction of enzymatic activity by more than 80%, suggesting that these conserved residues are located near the catalytic sites, where they coordinate the heme group and define the shape and size of the catalytic site. Planta 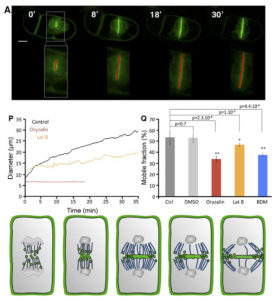 The cell plate is a common feature of plant cell divisions, but the mechanisms forming this structure are not completely understood. The cell plate is made from membrane vesicles carrying polysaccharide cargo, and thousands of tiny vesicles fuse together to form one cell plate. Observing fluorescence micrographs alone cannot provide a full picture of cell plate biogenesis, so Oostende-Triplet et al. developed FluMOS (Fluorescence Morphological Operators Software). This program can identify and analyze cell plates within fluorescence confocal images. The authors identified three distinct phases of cell plate expansion in N. tabacum BY-2 cells based on the growth rate of the cell plate. FluMOS quantitated vesicle movement towards nascent cell plates. Initially, the vesicles move quickly to the cell plate when its growth begins. As the cell plate growth, vesicle movement remains high at the edges, but vesicle movement slows in the center of the cell plate. Pharmacological inhibition of membrane trafficking and protein synthesis showed both processes contribute to cell plate expansion. (Summary by
The cell plate is a common feature of plant cell divisions, but the mechanisms forming this structure are not completely understood. The cell plate is made from membrane vesicles carrying polysaccharide cargo, and thousands of tiny vesicles fuse together to form one cell plate. Observing fluorescence micrographs alone cannot provide a full picture of cell plate biogenesis, so Oostende-Triplet et al. developed FluMOS (Fluorescence Morphological Operators Software). This program can identify and analyze cell plates within fluorescence confocal images. The authors identified three distinct phases of cell plate expansion in N. tabacum BY-2 cells based on the growth rate of the cell plate. FluMOS quantitated vesicle movement towards nascent cell plates. Initially, the vesicles move quickly to the cell plate when its growth begins. As the cell plate growth, vesicle movement remains high at the edges, but vesicle movement slows in the center of the cell plate. Pharmacological inhibition of membrane trafficking and protein synthesis showed both processes contribute to cell plate expansion. (Summary by  This week’s blog was written by Dr
This week’s blog was written by Dr