Getting It Done On Time: How Maize Orders DNA Replication
Wear et al. examine replication programs in plants. The Plant Cell 2017. https://doi.org/10.1105/tpc.17.00037
By William Thompson, Emily Wear, and Linda Hanley-Bowdoin
DNA replication is fundamental to all life, as it is the process by which genetic material is duplicated so it can be passed from cell to cell. However, the process is complicated by the fact that higher organisms have lots of DNA in each cell. For example, a single nucleus from a single cell of a corn plant typically contains two sets of chromosomes, each of which contains over 2 billion base pairs (“letters”) of DNA and over 30,000 genes. If the DNA from a single set of chromosomes could be unraveled and placed end to end, it would be over 2 feet long. To fit in the tiny nucleus of the cell, many times smaller than the tip of a human hair, all this DNA is compacted in various ways. However, DNA can’t be replicated in its compact state, so higher organisms have evolved sophisticated replication programs to unravel and replicate small segments of their chromosomes at different times.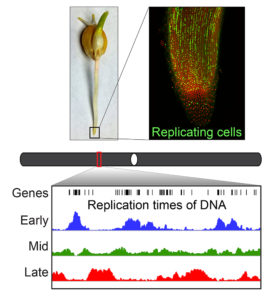
DNA replication is well characterized in animal cells, but little is known about replication programs in plants, which—until now—have not been studied with modern molecular techniques. Wear et al. describe the replication program in corn (aka maize, or Zea mays), the first time that replication of an entire genome has been characterized in any plant. It seems fitting that this feat was first accomplished in corn, because corn has been intensively studied by plant geneticists since early in the last century, and is now the most widely grown crop in the world.
The group’s observations show that the DNA close to the ends of the chromosome arms, where the genes are more densely packed, tend to replicate earlier, while the centers of the chromosomes, where there are fewer genes, tend to replicate later. This observation represents an important difference between corn and humans in the way the genes are organized and replication occurs. The authors also observed that the majority of genes that are highly expressed are replicating earlier. Knowing the time of replication for the entire corn genome represents an important contribution to our understanding of how processes in the nucleus can affect plant traits and their inheritance—knowledge that is increasingly crucial for modern plant improvement programs.
Plans for future research include a more detailed look at the molecular machinery that defines the maize replication program, and integrating laboratory experiments with computer modeling to understand the complex network of factors at work in the plant nucleus.
Wear, E.E., Song, J., Zynda, G., LeBlanc, C., Lee, T.-J., Mickelson-Young, L., Concia, L., Mulvaney, P., Szymanski, E.S., Allen, G.C., Martienssen, R., Vaughn, M.W., Hanley-Bowdoin, L., and Thompson, W. (2017). Genomic analysis of the DNA replication timing program during mitotic S phase in maize (Zea mays L.) root tips. Plant Cell Published August 25, 2017. DOI: https://doi.org/10.1105/tpc.17.00037.


 As I formally accepted the offer to join my Ph.D. program, the massive weight on my chest began to dissolve, my shoulders relaxed, and my headache subsided. Choosing a program had been quite the roller coaster ride, filled with uncertainty and sleepless nights. “Phew, well that’s finally over,” I said to myself. But just before that moment of relief, I felt a brief but intense wave of panic: Had I made the right choice? More than 4 years later, I think I chose well, and I hope that my difficult decision process offers some tips, and some solace, for others who are struggling with similar decisions. Looking back, I see that the key was realizing that it wasn’t enough for a program to meet my research interests; I needed to weigh factors outside the lab as well.
As I formally accepted the offer to join my Ph.D. program, the massive weight on my chest began to dissolve, my shoulders relaxed, and my headache subsided. Choosing a program had been quite the roller coaster ride, filled with uncertainty and sleepless nights. “Phew, well that’s finally over,” I said to myself. But just before that moment of relief, I felt a brief but intense wave of panic: Had I made the right choice? More than 4 years later, I think I chose well, and I hope that my difficult decision process offers some tips, and some solace, for others who are struggling with similar decisions. Looking back, I see that the key was realizing that it wasn’t enough for a program to meet my research interests; I needed to weigh factors outside the lab as well. Note – this article has a very useful set of suggestions and encouragements for writers, including “In science, sentences should be logical and unambiguous. You’re not writing literature, where ambiguity might be a good thing. There you might want two possible meanings on purpose. But in a scientific paper, you don’t want that. You want a very clear meaning. (Wim Vanderbauwhede, Computing Science, University of Glasgow)”
Note – this article has a very useful set of suggestions and encouragements for writers, including “In science, sentences should be logical and unambiguous. You’re not writing literature, where ambiguity might be a good thing. There you might want two possible meanings on purpose. But in a scientific paper, you don’t want that. You want a very clear meaning. (Wim Vanderbauwhede, Computing Science, University of Glasgow)”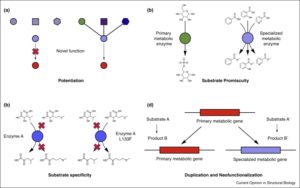 The huge set of chemical pathways beyond the conserved primary metabolic network is described as specialized metabolism (formerly known as secondary metabolism). The diversity of specialized metabolites is due to recent evolutionary innovations in enzyme function, as reviewed by Leong and Last. Key processes include enzyme and substrate promiscuity, changes in substrate specificity, and of course gene duplication and divergence. The authors provide examples of the involvement of these processes in the evolution of specialized metabolism. Case studies include: an enzyme involved in glucosinolate synthesis, BAHD acyltransferases, 4-coumarate:CoA ligase, and aromatic acid methyltransferases. Curr. Opin. Struct. Biol.
The huge set of chemical pathways beyond the conserved primary metabolic network is described as specialized metabolism (formerly known as secondary metabolism). The diversity of specialized metabolites is due to recent evolutionary innovations in enzyme function, as reviewed by Leong and Last. Key processes include enzyme and substrate promiscuity, changes in substrate specificity, and of course gene duplication and divergence. The authors provide examples of the involvement of these processes in the evolution of specialized metabolism. Case studies include: an enzyme involved in glucosinolate synthesis, BAHD acyltransferases, 4-coumarate:CoA ligase, and aromatic acid methyltransferases. Curr. Opin. Struct. Biol. 


