Photosynthesis in Desert Plants: It’s About Time
Boxall et al. investigate CAM photosynthesis in Kalanchoë fedtschenkoi The Plant Cell (2017). https://doi.org/10.1105/tpc.17.00301
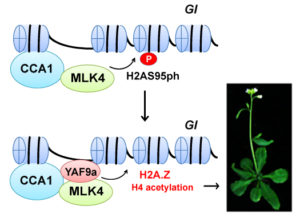
Background: During photosynthesis, most plants use the enzyme Rubisco to capture CO2 during the day. Crassulacean acid metabolism (CAM) plants such as prickly pears, pineapples, and agaves use a more efficient enzyme, phosphoenolpyruvate carboxylase (PEPC), to capture CO2 at night, and re-release the CO2 inside the leaf after sunrise. This allows them to close their leaf pores, and thus conserve water, during the hottest, driest part of day. We know relatively little about how CAM plants separate CO2 capture between the dark and the light. One key element, however, involves the PEPC regulator PPCK, which is made each night and helps PEPC to continue to capture CO2 in the face of rising levels of an inhibitor.
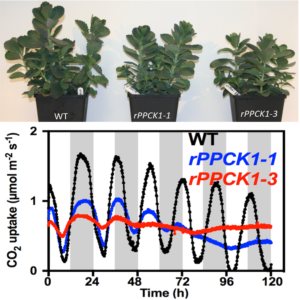
PPCK mutants (blue and red lines) are unable to regulate CO2 uptake with a circadian rhythm.
Question: We wanted to know if PPCK would be a necessary component of engineering other plants to use CAM photosynthesis. We tested this by switching the gene off in the CAM plant from Madagascar Kalanchoë fedtschenkoi (Lavender Scallops).
Findings: We found that, for CAM to work properly, the cells must switch on PPCK each night. When we prevented Kalanchoë from making PPCK at night, the plants could only capture a third of the CO2 captured by the normal plants. In addition, we discovered that the plants that were unable to make PPCK each night had alterations in their internal cellular timekeeping mechanism, the circadian clock. In CAM plants, the circadian clock optimizes CO2 fixation and PPCK is one of the key ways that the cellular clock communicates time signals to control the CAM process. What was surprising was that switching off PPCK led to changes in the circadian clock itself. We are now working to understand whether (and how) changes in cellular metabolism resulting from the drop in dark CO2 capture were communicated to the internal clock within the leaf cells where CAM occurs.
Next steps: Scientists aim to tweak crop plants to use the CAM system to produce new crops that are better suited to growth on drought-prone lands. Our work demonstrates that ongoing efforts to engineer CAM photosynthesis into other plants will need to introduce PPCK to protect PEPC from inhibition. With PPCK working the night shift, CAM works three times better, which should help crop scientists to make the most of this amazingly water-wise form of photosynthesis.
Susanna F. Boxall, Louisa V. Dever, Jana Knerova, Peter D. Gould and James Hartwell. (2017). Phosphorylation of Phosphoenolpyruvate Carboxylase is Essential for Maximal and Sustained Dark CO2 Fixation and Core Circadian Clock Operation in the Obligate Crassulacean Acid Metabolism Species Kalanchoë fedtschenkoi. https://doi.org/10.1105/tpc.17.00301


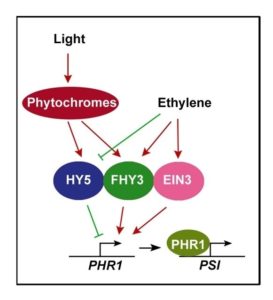 root system architecture (stopping primary root growth and increasing root hair and lateral root formation), reducing photosynthesis, enhancing Pi transporter activity, and accumulating starch and the pigment anthocyanin. How these activities are orchestrated is not yet well known. Arabidopsis PHOSPHATE STARVATION RESPONSE1 (PHR1) encodes a conserved transcription factor that helps program Pi starvation-induced gene expression and the resulting downstream Pi starvation responses. However, PHR1 expression is only weakly responsive to Pi deprivation stress. In this work, Liu et al. (2017) show that PHR1 expression is induced by light in a manner dependent on light receptors known as phytochromes.
root system architecture (stopping primary root growth and increasing root hair and lateral root formation), reducing photosynthesis, enhancing Pi transporter activity, and accumulating starch and the pigment anthocyanin. How these activities are orchestrated is not yet well known. Arabidopsis PHOSPHATE STARVATION RESPONSE1 (PHR1) encodes a conserved transcription factor that helps program Pi starvation-induced gene expression and the resulting downstream Pi starvation responses. However, PHR1 expression is only weakly responsive to Pi deprivation stress. In this work, Liu et al. (2017) show that PHR1 expression is induced by light in a manner dependent on light receptors known as phytochromes.