On March 24, the U.S. Department of Agriculture (USDA) Agriculture and Food Research Initiative (AFRI) published a request for applications (RFA) for the AFRI Water for Food Production Systems Challenge Area, which is a combination of the Food Security and Water for Agriculture Challenge Areas. This interdisciplinary initiative looks to address the issues of water availability and food production through the strategic management planning and the integration of new technologies. With long-term food security as the objective, projects are “expected to pursue greater savings in water use by realizing the optimal potentials of innovative management strategies and interventions that use appropriate technologies to exploit potential synergies across production systems and competing users.”
USDA requires that potential projects be integrated, including a research component as well as additional extension or education components. Priority will be given to projects which consider the tertiary benefits to wildlife, fish, and pollinator populations. Successful projects must address at least one of the following priority areas:
- “Use of transformative discoveries such as classical/conventional breeding as well as genomics, nanotechnology, sensors, modeling, microbiome manipulation, and data-driven decision tools to develop drought- and flood-tolerant cultivars, intensify food production, improve crop and livestock health, or reduce overall water use across food production systems;
- Innovative ways to sustainably secure and more efficiently use water to produce food given competing resource demands, varying annual and within season availability, and compromised or limited water quality;
- Targeted activities/interventions beyond simple information delivery to overcome barriers, disincentives, and institutional or legal impediments so that more sustainable management practices are adopted and acceptance encouraged;
- Safe use of non-traditional water sources such as agricultural runoff, recycled, treated, produced, and brackish waters as appropriate across food system supply chains, including production, processing, distribution, and consumption, so that total amounts of fresh water used are reduced.”
Award Information: USDA anticipates distributing approximately $34 million for 7 Coordinated Agricultural Projects (CAP) and Strengthening (Food and Agricultural Science Enhancement) CAP awards, with a maximum funding amount of $5.2 million per project. The maximum project duration under this RFA is five years.
Deadlines: The deadline for the submission of Letters of Intent is May 17, 2017. The application deadline is August 2, 2017.
Sources and additional information: https://nifa.usda.gov/sites/default/files/rfa/FY2017_AFRI_Water_for%20Food%20Production%20Systems.pdf
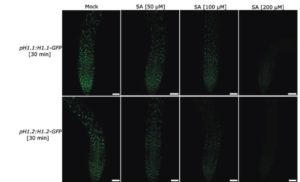 Green Fluorescent Protein (GFP) is a widely-used reporter with which to analyze protein localization and expression levels. De Jonge et al. report that GFP fluorescence is greatly diminished in the presence of the hormone salicylic acid (SA), as is the fluorescence of the reporters RFP and VENUS. The reduction of fluorescence is observed with many different promoters, suggesting that it not due to transcriptional effects. Furthermore, the protein level is not affected by SA, indicating again that it is not due to expression-level effects. Finally, the authors showed that the fluorescence in vitro is unaffected by SA, which suggests that the fluorescence reduction requires as yet undetermined cellular components. Until this effect is understood, it is a good idea to be cautious when using GFP or other similar markers in the presence of SA. J. Exp. Bot. 10.1093/jxb/erx031
Green Fluorescent Protein (GFP) is a widely-used reporter with which to analyze protein localization and expression levels. De Jonge et al. report that GFP fluorescence is greatly diminished in the presence of the hormone salicylic acid (SA), as is the fluorescence of the reporters RFP and VENUS. The reduction of fluorescence is observed with many different promoters, suggesting that it not due to transcriptional effects. Furthermore, the protein level is not affected by SA, indicating again that it is not due to expression-level effects. Finally, the authors showed that the fluorescence in vitro is unaffected by SA, which suggests that the fluorescence reduction requires as yet undetermined cellular components. Until this effect is understood, it is a good idea to be cautious when using GFP or other similar markers in the presence of SA. J. Exp. Bot. 10.1093/jxb/erx031 

 Monitoring cells and their activities in real time provides exceptional insights into their mechanisms and strategies of growth. Prunet has used live confocal imaging of Arabidopsis flowers to study the placement of floral organs and the dynamics of floral stem cells. Here, he presents a detailed protocol for live confocal imaging of developing Arabidopsis flowers. The included video will help new imagers learn the fine points of this powerful method. JOVE
Monitoring cells and their activities in real time provides exceptional insights into their mechanisms and strategies of growth. Prunet has used live confocal imaging of Arabidopsis flowers to study the placement of floral organs and the dynamics of floral stem cells. Here, he presents a detailed protocol for live confocal imaging of developing Arabidopsis flowers. The included video will help new imagers learn the fine points of this powerful method. JOVE 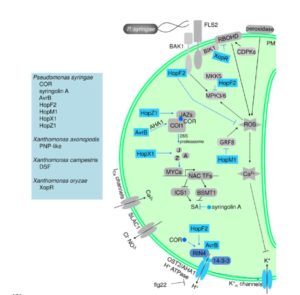 Stomatal defense, recognition of pathogens at the stomatal pore accompanied by stomatal closure to prevent their entry, was discovered ten years ago. Melotto et al. review what we’ve learned in the past decade about this key defense strategy. They discuss pathogen recognition, in which microbe-associated molecular patterns (MAMPs) are perceived by pattern recognition receptors (PRRs); downstream signaling including the contributions of ABA, salicylates and jasmonates; and the channels that mediate stomatal closure. They also review strategies by which pathogens are able to evade or suppress stomatal defenses, and the impact of environmental conditions on stomatal defenses. Plant Physol.
Stomatal defense, recognition of pathogens at the stomatal pore accompanied by stomatal closure to prevent their entry, was discovered ten years ago. Melotto et al. review what we’ve learned in the past decade about this key defense strategy. They discuss pathogen recognition, in which microbe-associated molecular patterns (MAMPs) are perceived by pattern recognition receptors (PRRs); downstream signaling including the contributions of ABA, salicylates and jasmonates; and the channels that mediate stomatal closure. They also review strategies by which pathogens are able to evade or suppress stomatal defenses, and the impact of environmental conditions on stomatal defenses. Plant Physol.  Beneficial microbes help plants take up nutrients, confer protection against pathogens, and can even affect flowering time. Busby et al. argue for a coordinated effort between researchers and farmers to study plant microbiomes with the goal of using them to enhance productivity. The authors define and describe five research priorities: 1. Develop model host–microbiome systems for crop plants and non-crop plants with associated microbial culture collections and reference genomes, 2. Define core microbiomes and metagenomes in model host–microbiome systems, 3. Elucidate the rules of synthetic, functionally programmable microbiome assembly, 4. Determine functional mechanisms of plant–microbiome interactions, 5. Characterize and refine plant genotype-by-environment-by-microbiome-by-management interactions. Each of these priorities is described further in the article. PLOS Biol.
Beneficial microbes help plants take up nutrients, confer protection against pathogens, and can even affect flowering time. Busby et al. argue for a coordinated effort between researchers and farmers to study plant microbiomes with the goal of using them to enhance productivity. The authors define and describe five research priorities: 1. Develop model host–microbiome systems for crop plants and non-crop plants with associated microbial culture collections and reference genomes, 2. Define core microbiomes and metagenomes in model host–microbiome systems, 3. Elucidate the rules of synthetic, functionally programmable microbiome assembly, 4. Determine functional mechanisms of plant–microbiome interactions, 5. Characterize and refine plant genotype-by-environment-by-microbiome-by-management interactions. Each of these priorities is described further in the article. PLOS Biol. 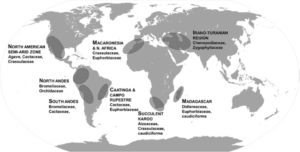 “Succulence is a phenomenon that has long eluded a decisive consensus definition,” begins Males in his review of the physiology and evolutionary developmental biology of succulence. Succulence can broadly be defined as the storage of water such that the plant can maintain physiological activity in the absence of an external supply (in other words, drought avoidance), but where and how this water is stored is highly variable. Furthermore, succulence has evolved many times independently, so the trait of succulence is quite diverse. The author describes the variables that affect the physiology of succulent water use, which include specialized cells and tissues, short-lived roots, and the dynamic regulation of aquaporins, as well as efforts to map these traits to genes, and the “complex relationship” between succulence and CAM, the specialized form of photosynthesis found in many but not all succulent plants. J. Exp. Bot.
“Succulence is a phenomenon that has long eluded a decisive consensus definition,” begins Males in his review of the physiology and evolutionary developmental biology of succulence. Succulence can broadly be defined as the storage of water such that the plant can maintain physiological activity in the absence of an external supply (in other words, drought avoidance), but where and how this water is stored is highly variable. Furthermore, succulence has evolved many times independently, so the trait of succulence is quite diverse. The author describes the variables that affect the physiology of succulent water use, which include specialized cells and tissues, short-lived roots, and the dynamic regulation of aquaporins, as well as efforts to map these traits to genes, and the “complex relationship” between succulence and CAM, the specialized form of photosynthesis found in many but not all succulent plants. J. Exp. Bot.  Roots are extraordinary examples of developmental plasticity, and hundreds of papers have reported on the mechanisms by which root system architecture responds to the environment and the global nutritional status of the plant. McCleery et al. propose a model that synthesizes evidence of intrinsic, local and long-distance signals. They suggest that the root system is best represented as a set of rhizomers (analogous to the phytomers that compose the shoot system), and that each rhizomer perceives and processes input (including hormones, peptides, miRNAs, and nutrients) to promote or suppress its developmental journey to lateral root emergence. Curr. Opin. Cell Biol.
Roots are extraordinary examples of developmental plasticity, and hundreds of papers have reported on the mechanisms by which root system architecture responds to the environment and the global nutritional status of the plant. McCleery et al. propose a model that synthesizes evidence of intrinsic, local and long-distance signals. They suggest that the root system is best represented as a set of rhizomers (analogous to the phytomers that compose the shoot system), and that each rhizomer perceives and processes input (including hormones, peptides, miRNAs, and nutrients) to promote or suppress its developmental journey to lateral root emergence. Curr. Opin. Cell Biol. 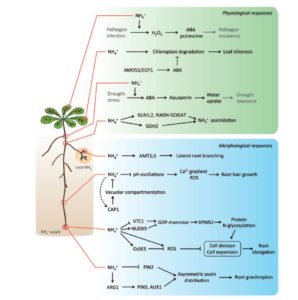 Ammonium is one of the major forms in which nitrogen is assimilated. Besides being a nutrient, it also acts as signal that affects gene expression and root system architecture. Some ammonium-induced genes are also induced by low pH (ammonium acidifies the apoplast), whereas others are specifically induced by ammonium or its assimilation products (e.g., glutamine). Liu and von Wirén review the molecular components of ammonium signaling, which include the AMT ammonium transporters/transceptors. Although many components have been identified, many gaps in our understanding remain and are discussed. J Exp. Bot.
Ammonium is one of the major forms in which nitrogen is assimilated. Besides being a nutrient, it also acts as signal that affects gene expression and root system architecture. Some ammonium-induced genes are also induced by low pH (ammonium acidifies the apoplast), whereas others are specifically induced by ammonium or its assimilation products (e.g., glutamine). Liu and von Wirén review the molecular components of ammonium signaling, which include the AMT ammonium transporters/transceptors. Although many components have been identified, many gaps in our understanding remain and are discussed. J Exp. Bot. 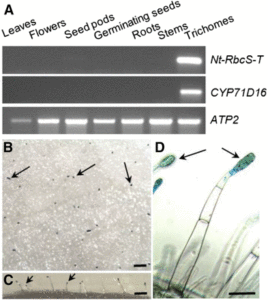 Rubisco is responsible for CO2 fixation during photosynthesis. This enzyme is assembled from eight large subunits (RbcL), encoded by a single chloroplast gene, and eight small subunits (RbcS), encoded by a nuclear gene family. Although Rubisco’s catalytic reaction is mostly controlled by the large subunit, the small subunit also positively influences the reaction. In vascular plants, Rubisco is primarily found in the photosynthetic ground tissue of leaves and stems. In certain species, however, photosynthesis also takes place in the secretory cells of glandular trichomes, epidermal outgrowths (hairs) involved in the secretion of specialized metabolites. In general, little is known about photosynthesis in trichomes, although its main function may be to help provide the carbon and energy needs for specialized metabolite production. Laterre et al. (
Rubisco is responsible for CO2 fixation during photosynthesis. This enzyme is assembled from eight large subunits (RbcL), encoded by a single chloroplast gene, and eight small subunits (RbcS), encoded by a nuclear gene family. Although Rubisco’s catalytic reaction is mostly controlled by the large subunit, the small subunit also positively influences the reaction. In vascular plants, Rubisco is primarily found in the photosynthetic ground tissue of leaves and stems. In certain species, however, photosynthesis also takes place in the secretory cells of glandular trichomes, epidermal outgrowths (hairs) involved in the secretion of specialized metabolites. In general, little is known about photosynthesis in trichomes, although its main function may be to help provide the carbon and energy needs for specialized metabolite production. Laterre et al. (