Clathrin and Stomatal Function
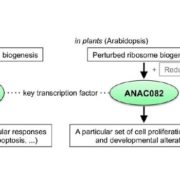
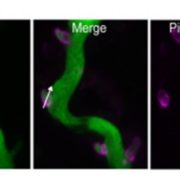
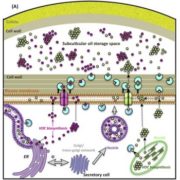
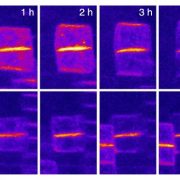
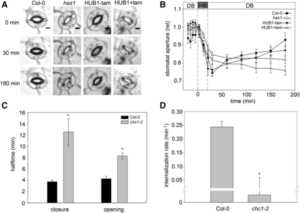 Vesicle traffic to and from the plasma membrane plays an integral role in regulating protein localization and activity, membrane composition, and cell surface area. Clathrin is a structural protein that forms a lattice-like complex composed of two H chain subunits (CHC1 and CHC2) and two light chain subunits (CLC1 and CLC2). The subunits assemble a “cage” around developing vesicles to support vesicle formation at the plasma membrane and endosomal membranes in plants and animals. This scaffolding dissociates once the vesicle is fully formed. Although a reduction in endocytosis has been noted in the roots of chc2 mutants, the known single chc mutant alleles do not present severe phenotypes, suggesting a redundancy in function of the subunits. Larson et al. (10.1104/pp.17.00970 ) have identified the has1 mutant, which has a stomatal function defect, as a clathrin heavy chain1 (CHC1) mutant allele. Stomata of the has1 mutant are more closed under dehydration stress conditions, indicating a new phenotype associated with the CHC1 mutant alleles and providing evidence for clathrin-mediated endocytosis in stomatal function. The has1 mutant also has a decreased rate of endocytosis and growth defects that are shared with other chc1 mutant alleles. The aberrant functions of chc mutant stomata are similar to those of the secretory SNARE mutant, syp121. The syp121 and chc mutants have impaired endo- and exocytosis compared to the wild type, indicating a link between SYP121-dependent secretion and clathrin-dependent endocytosis at the plasma membrane. These findings indicate that the functions of clathrin and SYP121 are important for the coordination of endo- and exocytosis and affect stomatal function, gas exchange, and vegetative growth in Arabidopsis.
Vesicle traffic to and from the plasma membrane plays an integral role in regulating protein localization and activity, membrane composition, and cell surface area. Clathrin is a structural protein that forms a lattice-like complex composed of two H chain subunits (CHC1 and CHC2) and two light chain subunits (CLC1 and CLC2). The subunits assemble a “cage” around developing vesicles to support vesicle formation at the plasma membrane and endosomal membranes in plants and animals. This scaffolding dissociates once the vesicle is fully formed. Although a reduction in endocytosis has been noted in the roots of chc2 mutants, the known single chc mutant alleles do not present severe phenotypes, suggesting a redundancy in function of the subunits. Larson et al. (10.1104/pp.17.00970 ) have identified the has1 mutant, which has a stomatal function defect, as a clathrin heavy chain1 (CHC1) mutant allele. Stomata of the has1 mutant are more closed under dehydration stress conditions, indicating a new phenotype associated with the CHC1 mutant alleles and providing evidence for clathrin-mediated endocytosis in stomatal function. The has1 mutant also has a decreased rate of endocytosis and growth defects that are shared with other chc1 mutant alleles. The aberrant functions of chc mutant stomata are similar to those of the secretory SNARE mutant, syp121. The syp121 and chc mutants have impaired endo- and exocytosis compared to the wild type, indicating a link between SYP121-dependent secretion and clathrin-dependent endocytosis at the plasma membrane. These findings indicate that the functions of clathrin and SYP121 are important for the coordination of endo- and exocytosis and affect stomatal function, gas exchange, and vegetative growth in Arabidopsis.