A Once-Hidden ER Matrix Reveals the Totally Tubular Function of LUNAPARKs in Plants
The endoplasmic reticulum (ER) is an organelle that is vital for many cellular processes such as protein and lipid synthesis, calcium signaling, detoxification, and movement of other organelles. It forms an intricate meshwork in the cell cortex that is comprised of highly dynamic interconnected tubules and cisternae (“sheets”) that are continually rearranging. In plants, ROOT HAIR DEFECTIVE3 (RHD3), like its homologs in animals and yeast, is an ER membrane-localized GTPase and is one player involved in the tight regulation of ER shape that is required for its proper function and normal organismal development (Chen et al., 2011). Overexpression of these GTPases causes excessive fusion of ER tubules. In animals and yeast, the Lunapark (Lnp) protein seems to counteract their action by stabilizing ER tubules. However, the role of plant LUNAPARK (LNP) homologs was debated—until now.
Tubules and cisternae have long been viewed as diametrical but continuous and intermorphing structures of the ER, whose proportions change depending on developmental stage and in response to environmental stimuli. Compared to the wild type, lnp mutants display cortical ER that seems to have fewer sheets and larger intertubule spaces (lacunae), whereas overexpression of Arabidopsis (Arabidopsis thaliana) LNP1 or LNP2 in tobacco (Nicotiana tabacum) causes an increase in ER sheets. It was therefore hypothesized that LNPs raise the ratio of sheets to tubules (Kriechbaumer et al., 2018), a role opposite to that of the animal and yeast homologs. Additionally, because of the presence of large clumps of ER membrane in lnp mutants, it was hypothesized that LNPs play a role in the proper distribution of ER throughout the cell (Ueda et al., 2018).
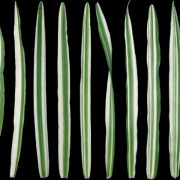
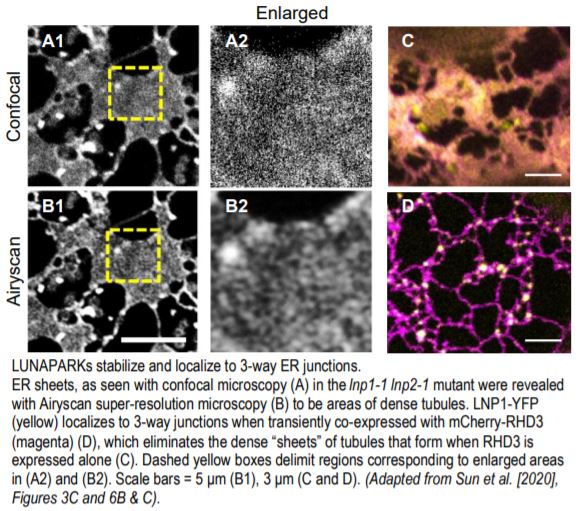 The controversy surrounding the function of LNPs in plants stemmed from the previously unrecognized and difficult to decipher morphology of the ER in lnp mutants. Drawing from the demonstration with super-resolution microscopy in animal cells that many ER sheets are actually clusters of dense tubules that form an “ER matrix” (Nixon-Abell et al., 2016), Sun et al. (2020) have now overcome this barrier by employing Airyscan super-resolution and transmission electron microscopy techniques. They established here that lnp mutants exhibit a densely packed ER network, both in the cytosolic ER clumps and in the areas once considered to be closed ER sheets (see figure).
The controversy surrounding the function of LNPs in plants stemmed from the previously unrecognized and difficult to decipher morphology of the ER in lnp mutants. Drawing from the demonstration with super-resolution microscopy in animal cells that many ER sheets are actually clusters of dense tubules that form an “ER matrix” (Nixon-Abell et al., 2016), Sun et al. (2020) have now overcome this barrier by employing Airyscan super-resolution and transmission electron microscopy techniques. They established here that lnp mutants exhibit a densely packed ER network, both in the cytosolic ER clumps and in the areas once considered to be closed ER sheets (see figure).
The authors also clarified the relationship between LNPs and RHD3. Although the phenotypes were not identical, the lnp1-1 lnp2-1 double mutant, like rhd3 mutants, had shorter root hairs and a smaller overall size compared to the wild type. In addition, interactions between RHD3 and LNP1 or LNP2 proteins were robustly shown using various methods. More importantly, the authors reconcile previous subcellular localization results and unveil a previously cryptic finding that, as in animals, LNPs do indeed localize to 3-way ER junctions—they just need to be in relatively equal proportions to RHD3 to do so.
When RHD3 levels were high, either endogenously in the lnp1-1 lnp2-1 mutant (as demonstrated via immunoblot analysis) or by overexpression, the ER formed a dense tubular network in the form of “sheets.” In lnp1-1 and lnp2-1 plants complemented with LNP1pro:LNP1-YFP and LNP2pro:LNP2-YFP, respectively, LNP1 or LNP2 was concentrated in punctae at 3-way junctions. By contrast, LNP1 or LNP2 was dispersed evenly throughout the ER tubules in the rhd3-8 mutant, as was LNP1-RFP or LNP2-RFP expressed transiently in tobacco. Most striking was what happened when either LNP-RFP was coexpressed with YFP-RHD3: (1) the ER resumed its normal shape, no longer presenting as dense tubular “sheets” as seen when RHD3 is overexpressed; and (2) the localization pattern of LNP1 or LNP2 became punctate and concentrated at 3-way junctions (see figure).
Interestingly, newly formed 3-way junctions devoid of LNP1-RFP were shorter lived than those with it, and the presence of LNP1-YFP caused the disappearance of mCherry-RHD3 concentrated at these junctions. Ubiquitination assays demonstrated that LNP1 had E3 ubiquitin ligase activity and caused an increase in RHD3 ubiquitination levels, which were lower in the lnp1-1 lnp2-1 mutant background despite a marked increase in the RHD3 protein itself.
These collective results evoke a model whereby RHD3 recruits LNPs to 3-way ER junctions through direct interaction, causing LNPs to ubiquitinate and thereby target RHD3 for degradation by both the 26S proteasome and selective autophagy. This stabilizes 3-way junctions, preventing excessive tubule formation. It now appears that LNPs are more like their animal and yeast counterparts than previously thought! Importantly, the finding that Arabidopsis LNPs act as E3 ligases that target RHD3 may shed light on how Lnp proteins in animals and yeast function.
Anne C. Rea
Michigan State University
MSU-DOE Plant Research Laboratory
ORCID: 0000-0002-2996-5709
REFERENCES
Chen, J., Stefano, G., Brandizzi, F., and Zheng, H. (2011). Arabidopsis RHD3 mediates the generation of the tubular ER network and is required for Golgi distribution and motility in plant cells. J. Cell Sci. 124: 2241–2252.
Kriechbaumer, V., Breeze, E., Pain, C., Tolmie, F., Frigerio, L., and Hawes, C. (2018). Arabidopsis Lunapark proteins are involved in ER cisternae formation. New Phytol. 219: 990–1004.
Nixon-Abell, J., Obara, C.J., Weigel, A.V., Li, D., Legant, W.R., Xu, C.S., Pasolli, H.A., Harvey, K., Hess, H.F., Betzig, E., Blackstone, C., and Lippincott-Schwartz, J. (2016). Increased spatiotemporal resolution reveals highly dynamic dense tubular matrices in the peripheral ER. Science 354: aaf3928.
Sun, J., Movahed, N., and Zheng, H. (2020). Lunapark is a novel E3 ligase that mediates degradation of RHD3 to maintain a tubular ER network in Arabidopsis. Plant Cell DOI: https://doi.org/10.1105/tpc.18.00973
Ueda, H., Ohta, N., Kimori, Y., Uchida, T., Shimada, T., Tamura, K., and Hara-Nishimura, I. (2018). Endoplasmic reticulum (ER) membrane proteins (LUNAPARKs) are required for proper configuration of the cortical ER network in plant cells. Plant Cell Physiol. 59: 1931–1941.