Hold Me, Fold Me…or Not!
It’s not just human relationships that may require a chaperone to prevent inappropriate interactions. Numerous proteins in organisms from Escherichia coli to us, especially hydrophobic membrane proteins, also require chaperones in aqueous environments to prevent inappropriate interactions such as aggregation or improper folding (Gruber and Horovitz, 2016).
Whereas most plasma membrane proteins in E. coli are inserted cotranslationally by ribosomes attached to the target membrane, thylakoid membrane proteins in the chloroplast are typically nucleus-encoded, translated in the cytoplasm, and must be inserted into thylakoid membranes posttranslationally. After crossing the chloroplast outer and inner membranes, they must transit the aqueous stroma en route to the thylakoids, often requiring chaperones (Zhao and Liu, 2018). Also, for proper targeting, a particular thylakoid membrane protein may need to be either folded or unfolded.
 Cpn60 is a chloroplast chaperone protein involved in folding of stromal proteins (Kim et al., 2013). It is part of a 700-kD protein complex that dissociates upon ATP addition, as is typical of chaperonin-substrate complexes. Using the thylakoid protein Plastidic type I signal peptidase 1 (Plsp1) as a model substrate for insertion into the thylakoid membrane, Klasek et al. (2020) now ask: Does Cpn60 facilitate proper integration of Plsp1 into the thylakoid membrane and, if so, how? Because premature folding of Plsp1 in the stroma results in mistargeting in the thylakoid membrane (Endow et al., 2015), they tested whether integration was influenced by the ratio of Cpn60 to cpSecA1, the stromal motor of the cpSec1 translocon that inserts unfolded Plsp1 into the thylakoid membrane.
Cpn60 is a chloroplast chaperone protein involved in folding of stromal proteins (Kim et al., 2013). It is part of a 700-kD protein complex that dissociates upon ATP addition, as is typical of chaperonin-substrate complexes. Using the thylakoid protein Plastidic type I signal peptidase 1 (Plsp1) as a model substrate for insertion into the thylakoid membrane, Klasek et al. (2020) now ask: Does Cpn60 facilitate proper integration of Plsp1 into the thylakoid membrane and, if so, how? Because premature folding of Plsp1 in the stroma results in mistargeting in the thylakoid membrane (Endow et al., 2015), they tested whether integration was influenced by the ratio of Cpn60 to cpSecA1, the stromal motor of the cpSec1 translocon that inserts unfolded Plsp1 into the thylakoid membrane.
Plsp1 is also found in a 700-kD complex in the stroma, so the authors tested whether Plsp1 bound directly to Cpn60. In vitro pull-down assays showed that Plsp1 bound specifically to Cpn60 and pulled it down in stoichiometric amounts. Cpn60 also protected Plsp1 from proteinase K digestion. More detailed proteomic analysis showed that Plsp1 interacted with specific α and β isoforms of Cpn60, but not others, suggesting substrate specificity of the different isoforms.
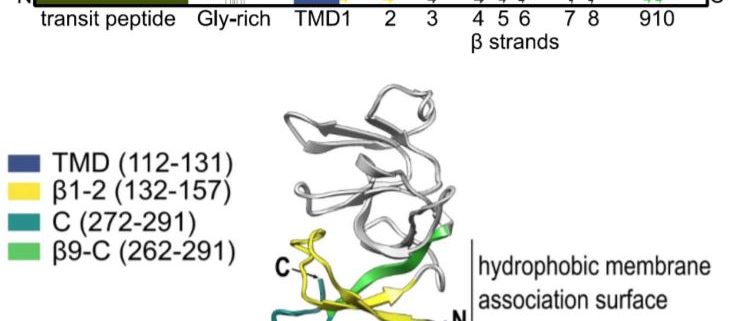
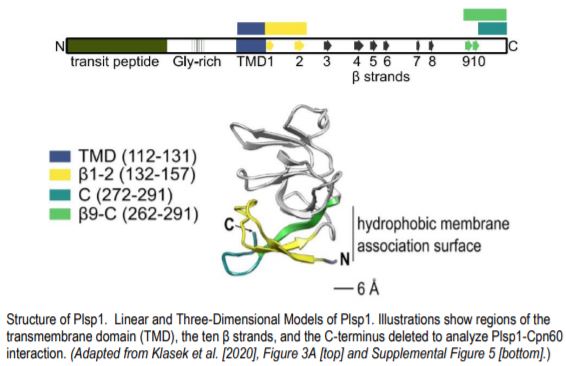
After showing that Plsp1 physically interacts with Cpn60, the authors demonstrated that Cpn60 and cpSec1 interact with adjacent amino acid residues of Plsp1. Analysis of a series of deletion constructs revealed that specific β strands adjacent to the transmembrane domain were important for interaction with Cpn60 (see figure). Removal of these residues abolished integration.
Using radiolabeled Plsp1 translated in vitro and Cpn60α1 and β1 isolated from pea (Pisum sativum), they reconstituted the 700-kD complex and, like the native complex, it disappeared upon treatment with ATP. Then, using an import-chase assay to introduce a pulse of Plsp1 into isolated chloroplasts, they found that the abundance of the 700-kD complex containing Cpn60 decreased as the amount of Plsp1 inserted into the membrane increased. Addition of denatured Rubisco (a Cpn60 substrate) to a Cpn60-Plsp1 complex displaced Plsp1 from Cpn60, presumably making it more accessible to the cpSec1 translocon and restoring integration of Plsp1 into the thylakoid membrane by the cpSec1 pathway.
The authors conclude by presenting three plausible models to describe how Cpn60 “hands off” Plsp1 to the cpSecI translocon. They show how their data support the model in which Cpn60 binds and releases Plsp1 in cycles as Plsp1 transits the stroma en route to cpSec1 and its final destination in the thylakoid membrane. Cpn60 shields the hydrophobic membrane protein from potential aggregation in the aqueous stroma and, like a human chaperone, prevents unwanted and potentially detrimental interactions.
Gregory Bertoni
Science Editor
ORCID ID: 0000-0001-7977-3724
REFERENCES
Endow, J.K., Singhal, R., Fernandez, D.E., and Inoue, K. (2015). Chaperone-assisted post-translational transport of plastidic type I signal peptidase 1. J. Biol. Chem. 290, 28778-28791.
Gruber, R., and Horovitz, A. (2016). Allosteric Mechanisms in Chaperonin Machines. Chem. Rev. 116, 6588-6606.
Kim, S.-R., Yang, J.-I., and An, G. (2013). OsCpn60α1, encoding the plastid chaperonin 60α subunit, is essential for folding of rbcL. Molecules and Cells 35, 402-409.
Klasek, L., Inoue, K, and Theg, S. M. (2020). Chloroplast chaperonin-mediated targeting of a thylakoid membrane protein. Plant Cell. Published Dec. 2020. DOI: https://doi.org/10.1105/tpc.20.00309.
Zhao, Q., and Liu, C. (2018). Chloroplast Chaperonin: An Intricate Protein Folding Machine for Photosynthesis. Frontiers in Molecular Biosciences 4:98.