Metallic Action! The Dynamics of a Tripartite Iron Uptake Complex in Arabidopsis Roots
Metallic action! The dynamics of a tripartite iron uptake complex in Arabidopsis roots
By Marcelo Lattarulo Campos
Integrative Plant Research Laboratory, Departamento de Botânica e Ecologia, Instituto de Biociências, Universidade Federal de Mato Grosso, Cuiabá/MT, Brazil.
ORCID ID: 0000-0001-6235-5120
Twitter: @lattarulo
Plants generally obtain mineral nutrients from the soil through their roots; however, the availability of a specific soil nutrient may limit plant growth and productivity and, conversely, the presence of too much of a specific soil nutrient may be toxic to the plant. Iron (Fe) is an essential micronutrient that fulfils numerous roles in plant cellular processes, serving as a key component for respiration and photosynthesis and as a cofactor for many enzymes (Barker & Pilbeam, 2015). Although relatively abundant in the Earth’s crust, iron is normally found in soils in its ferric form (Fe3+), which is poorly bioavailable due to its low solubility. To cope with this difficulty, non-gramineous plants like the model plant Arabidopsis thaliana have evolved a Fe3+ reduction-based mechanism that involves acidification of the rhizosphere by the proton pump H+-ATPASE2 (AHA2), reduction and solubilization of Fe3+ to Fe2+ by FERRIC REDUCTION OXIDASE2 (FRO2), and finally transport of Fe2+ into the cell by IRON REGULATED TRANSPORTER1 (IRT1) (Jeong et al., 2017; Rajniak et al., 2018). In addition to acting as the high affinity iron uptake transporter (Jeong et al., 2017), IRT1 also acts as a broad-spectrum transporter, mediating the absorption of a variety of metals such as cadmium, cobalt, and zinc. Plants alter the expression, subcellular localization and turnover of IRT1 to finely tune the uptake of iron and other metals from the soil (Cointry & Vert, 2019). Surprisingly, aside from the importance of IRT1 for metal homeostasis, little is known about other proteins associated with IRT1 dynamics and activity.
 In this issue of Plant Physiology, Martín-Barranco et al., (2020) shed light on this topic by exploring the interactome of IRT1 in Arabidopsis root epidermal cells. To co-purify IRT-associated proteins, they used an Arabidopsis irt1-1 null mutant expressing an IRT1-mCitrine fusion protein under the control of the IRT1 promoter. To induce IRT1-mCitrine expression, transgenic plants were grown on media containing iron, and then transferred to media with no iron, but containing an excess of non-iron metal substrates (Zn, Mn and Co). Root protein extracts were used to immunopurify IRT1-mCitrine and its interacting proteins, which were then analyzed by mass spectrometry. Among the 142 putative interacting proteins identified, two were particularly notable for their participation with IRT1 in the acidification-reduction-transport of iron uptake in Arabidopsis: AHA2 and FRO2. These results were confirmed by co-immunoprecipitation and split-ubiquitin assays, providing strong evidence that IRT1 directly interacts with AHA2 and FRO2. Moreover, these experiments also revealed that AHA2 and FRO2 physically associate with each other. Taken together, these observations indicate the existence of an IRT1-AHA2-FRO2 tripartite protein complex that modulates iron uptake in Arabidopsis root epidermal cells.
In this issue of Plant Physiology, Martín-Barranco et al., (2020) shed light on this topic by exploring the interactome of IRT1 in Arabidopsis root epidermal cells. To co-purify IRT-associated proteins, they used an Arabidopsis irt1-1 null mutant expressing an IRT1-mCitrine fusion protein under the control of the IRT1 promoter. To induce IRT1-mCitrine expression, transgenic plants were grown on media containing iron, and then transferred to media with no iron, but containing an excess of non-iron metal substrates (Zn, Mn and Co). Root protein extracts were used to immunopurify IRT1-mCitrine and its interacting proteins, which were then analyzed by mass spectrometry. Among the 142 putative interacting proteins identified, two were particularly notable for their participation with IRT1 in the acidification-reduction-transport of iron uptake in Arabidopsis: AHA2 and FRO2. These results were confirmed by co-immunoprecipitation and split-ubiquitin assays, providing strong evidence that IRT1 directly interacts with AHA2 and FRO2. Moreover, these experiments also revealed that AHA2 and FRO2 physically associate with each other. Taken together, these observations indicate the existence of an IRT1-AHA2-FRO2 tripartite protein complex that modulates iron uptake in Arabidopsis root epidermal cells.
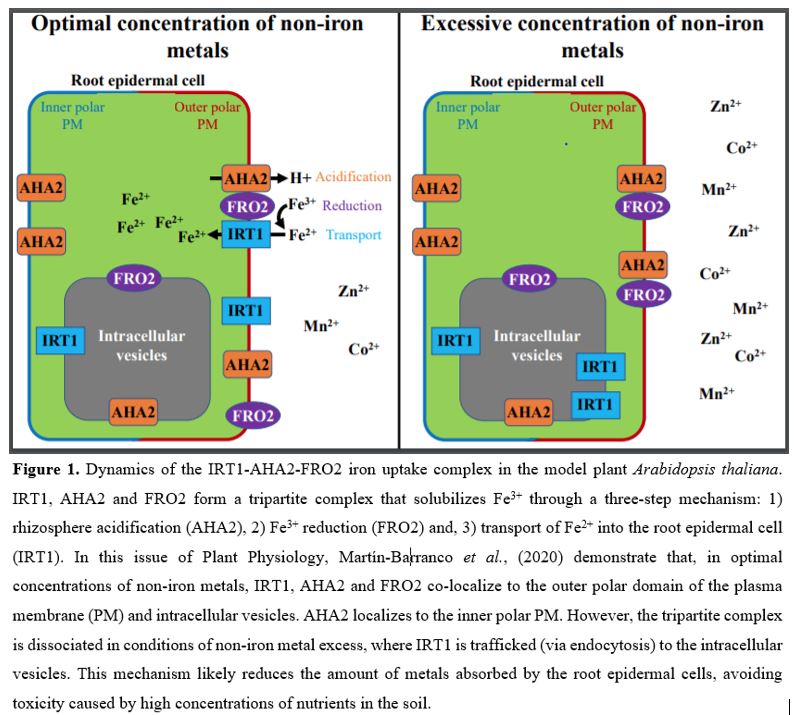
High concentrations of mineral nutrients outside and inside plant cells can lead to toxicity due to oxidative and osmotic stress, and thus hinder plant growth and development. As a possible mechanism to protect plants from toxic concentration of metal nutrients, IRT1 is polyubiquitinated in the presence of non-iron metal excess, leading to its removal from the plasma membrane and degradation in the vacuole (Dubeaux et al., 2018). As AHA2 and FRO2 belong to the same IRT1 protein complex, Martín-Barranco et al., (2020) wondered whether these two proteins were also ubiquitinated under conditions of high availability of non-iron metals. Using anti-ubiquitin immunoblots, the authors observed that whereas the excess of non-iron metals lead to a strong increase in IRT1 ubiquitin levels, the pools of ubiquitinated AHA2 and FRO2 remain unchanged when plants were treated with optimal and high concentration of these nutrients. These results provide striking evidence that, despite belonging to the same protein complex and participating in a common physiological mechanism (i.e. iron uptake), IRT1, AHA2 and FRO2 are differentially regulated by metal availability.
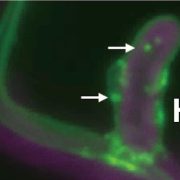
To better understand the intracellular dynamics of the IRT-AHA2-FRO2 complex, Martín-Barranco et al., (2020) evaluated the subcellular localization of these three proteins in the presence of metal nutrients. For this purpose, plants expressing fluorescently tagged versions of IRT, AHA2 and FRO2 were subjected to treatment with non-iron metal substrates, followed by visualization of root tip epidermal cells by confocal microscopy. The authors observed that, in the presence of physiologically relevant amounts of non-iron metal substrates, IRT1 and FRO2 co-localize at the outer polar domain of the plasma membrane (facing the rhizosphere) and in intracellular vesicles (early endosomes). Under these same conditions, AHA2 displayed an apolar localization to the plasma membrane, with weak fluorescent signals in the intracellular vesicles. Upon treatment with an excess of non-iron metals, IRT1 was depleted from the cell surface and accumulated in the intracellular vesicles. By contrast, treatment with excess non-iron metals did not change the localization of AHA2 and FRO2, which still mostly localized at the plasma membrane and in the intracellular vesicles (Fig. 1). These results indicate that, distinct from IRT1, AHA2 and FRO2 are not endocytosed in response to high concentrations of metal nutrients, suggesting that the IRT1-AHA2-FRO2 complex dissociates prior to IRT1 endocytosis and that the members of the tripartite complex are differentially regulated by metal availability.
The capacity of plants to regulate nutrient absorption is of great interest, since it has a major impact on crop productivity. Boosting mineral nutrients with fertilizers to maintain plant growth carries high environmental risk due to soil salinization and nutrient leakage (Barker & Pilbeam, 2015). Therefore, the development of crops that are more efficient at absorbing nutrients is imperative. The work by Martín-Barranco et al., (2020) describes, at the molecular level, how plants finely tune the uptake of essential metal nutrients in different conditions of availability. Although future work is needed to precisely determine how the activity of the IRT1-AHA2-FRO2 complex may influence the activity of each of its members and how it is optimized for nutrient uptake in plants, this study provides useful information for efforts to improve plant nutrient efficiency.
LITERATURE CITED
Barker AV, Pilbeam DJ (2015). Handbook of plant nutrition. Boca Raton, FL, USA: Taylor & Francis Group.
Cointry V, Vert G (2019). The bifunctional transporter-receptor IRT1 at the heart of metal sensing and signalling. New Phytol 223:1173-1178.
Dubeaux G, Neveu J, Zelazny E, Vert E (2018). Metal sensing by the IRT1 transporter-receptor orchestrates its own degradation and plant metal nutrition. Mol Cell 69:953-964.
Jeong J, Merkovich A, Clyne M, Connolly EL (2017). Directing iron transport in dicots: regulation of iron acquisition and translocation. Curr Opin Plant Biol 39:106-113.
Rajniak J, Giehl RFH, Chang E, Murgia I, Wirén N, Sattely ES (2018). Biosynthesis of redox-active metabolites in response to iron deficiency in plants. Nat Chem Biol 14:442-450.