Signaling secrets: FERONIA´s dynamic interplay with interactors at the cell surface
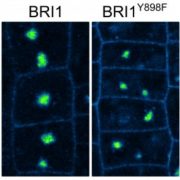
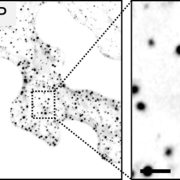
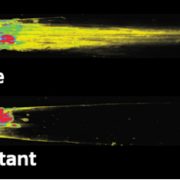
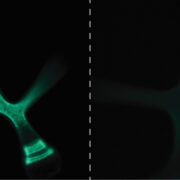
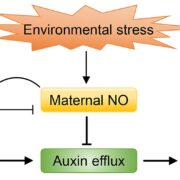
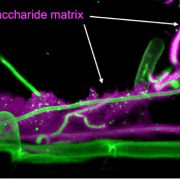
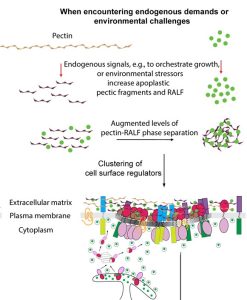 Named after the Etruscan goddess of fertility, the receptor-like kinase FERONIA (FER) is involved in more than fertility. A recent paper by Liu, Yeh et al. sheds light on the intricate signaling processes taking place on the cell surface in response to environmental signals regulating growth and development of plant cells. From previous studies, we know that FER can bind the cell wall polysaccharide pectin and interact with its peptide ligand, rapid alkalinization factor1 (RALF1). In this paper, the current signaling model is expanded. What is striking here is that the RALF1-FER interaction involves pectin but also LLG1 (LORELEI-LIKE glycosylphosphatidylinositol-anchored protein 1), a co-receptor of FER, guiding FER to its functional location. The molecular condensates formed by phase separation of RALF1 and pectin in the apoplastic space function as surface sensors for stress. The authors demonstrate that upon binding of the peptide ligand RALF1 at the plasma membrane, FER and LLG1 are internalized, concentrating in a nanodomain at the membrane. Interestingly, RALF1 itself does not get taken up into the cell, remaining in the apoplast. This interaction represents the initial step in a signal cascade in order to trigger downstream responses. The authors note that these condensates are further stimulated by environmental changes, activating a variety of downstream responses, including those associated with stresses such as salt and heat. This study highlights the connection between extracellular processes and intracellular responses implemented initially by upregulated endocytosis and expands our understanding of the diverse biological role of FER. (Summary by Ann-Kathrin Rößling @AK_Roessling) Cell 10.1016/j.cell.2023.11.038
Named after the Etruscan goddess of fertility, the receptor-like kinase FERONIA (FER) is involved in more than fertility. A recent paper by Liu, Yeh et al. sheds light on the intricate signaling processes taking place on the cell surface in response to environmental signals regulating growth and development of plant cells. From previous studies, we know that FER can bind the cell wall polysaccharide pectin and interact with its peptide ligand, rapid alkalinization factor1 (RALF1). In this paper, the current signaling model is expanded. What is striking here is that the RALF1-FER interaction involves pectin but also LLG1 (LORELEI-LIKE glycosylphosphatidylinositol-anchored protein 1), a co-receptor of FER, guiding FER to its functional location. The molecular condensates formed by phase separation of RALF1 and pectin in the apoplastic space function as surface sensors for stress. The authors demonstrate that upon binding of the peptide ligand RALF1 at the plasma membrane, FER and LLG1 are internalized, concentrating in a nanodomain at the membrane. Interestingly, RALF1 itself does not get taken up into the cell, remaining in the apoplast. This interaction represents the initial step in a signal cascade in order to trigger downstream responses. The authors note that these condensates are further stimulated by environmental changes, activating a variety of downstream responses, including those associated with stresses such as salt and heat. This study highlights the connection between extracellular processes and intracellular responses implemented initially by upregulated endocytosis and expands our understanding of the diverse biological role of FER. (Summary by Ann-Kathrin Rößling @AK_Roessling) Cell 10.1016/j.cell.2023.11.038