TTL bridges microtubules and cellulose synthase complexes
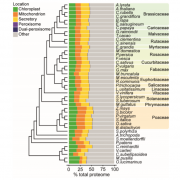
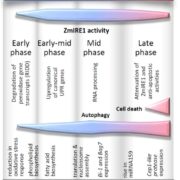
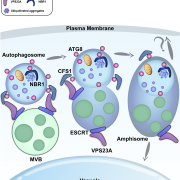
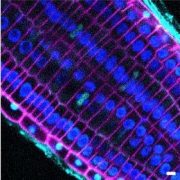
 Cellulose synthase (CESA) complexes (CSCs) synthesize the main polysaccharide component of plant primary cell wall, cellulose. The trafficking and dynamics of CSC are tightly regulated. Kesten et al. identified a new family of CSC- and microtubule-interacting proteins, named TETRATRICOPEPTIDE THIOREDOXIN-LIKE (TTL) proteins, which help to maintain cellulose synthesis under salt stress. The ttl1 ttl3 double mutant is hypersensitive to salt stress, and contains less cellulose content comparing to wild type under salt stress. The CSC foci density decreases more rapidly in ttl1 ttl3 double mutant upon salt stress, and fails to recover in 32 h, compared to wild type. And interestingly, TTL3 predominantly localizes to cytosolic regions under control conditions, but re-localizes to the plasma membrane (PM) upon salt stress, revealed by live-cell imaging and subcellular fractionation analysis. Additionally, TTL3 directly interacts with the cytosolic catalytic domain of CESA1. Live-cell imaging provides evidence that TTL3-GFP and tdTomato-CESA6 colocalize and comigrate at the PM, suggesting that the TTL protein might be a component of CSC. Furthermore, TTL3 can directly interact with microtubules. Taken together, TTL proteins might bridge salt stress perception with the dynamic control of cellulose synthesis at the PM. (Summary by Xiaohui Li @Xiao_hui_Li) Sci. Adv. 10.1126/sciadv.abq6971
Cellulose synthase (CESA) complexes (CSCs) synthesize the main polysaccharide component of plant primary cell wall, cellulose. The trafficking and dynamics of CSC are tightly regulated. Kesten et al. identified a new family of CSC- and microtubule-interacting proteins, named TETRATRICOPEPTIDE THIOREDOXIN-LIKE (TTL) proteins, which help to maintain cellulose synthesis under salt stress. The ttl1 ttl3 double mutant is hypersensitive to salt stress, and contains less cellulose content comparing to wild type under salt stress. The CSC foci density decreases more rapidly in ttl1 ttl3 double mutant upon salt stress, and fails to recover in 32 h, compared to wild type. And interestingly, TTL3 predominantly localizes to cytosolic regions under control conditions, but re-localizes to the plasma membrane (PM) upon salt stress, revealed by live-cell imaging and subcellular fractionation analysis. Additionally, TTL3 directly interacts with the cytosolic catalytic domain of CESA1. Live-cell imaging provides evidence that TTL3-GFP and tdTomato-CESA6 colocalize and comigrate at the PM, suggesting that the TTL protein might be a component of CSC. Furthermore, TTL3 can directly interact with microtubules. Taken together, TTL proteins might bridge salt stress perception with the dynamic control of cellulose synthesis at the PM. (Summary by Xiaohui Li @Xiao_hui_Li) Sci. Adv. 10.1126/sciadv.abq6971