Changing the Shape of the Apex via Cell-size Regulation
By Jiajun Wang, Ning Sun, Fangfang Zhang, Renbo Yu, Haodong Chen, Xing Wang Deng, and Ning Wei
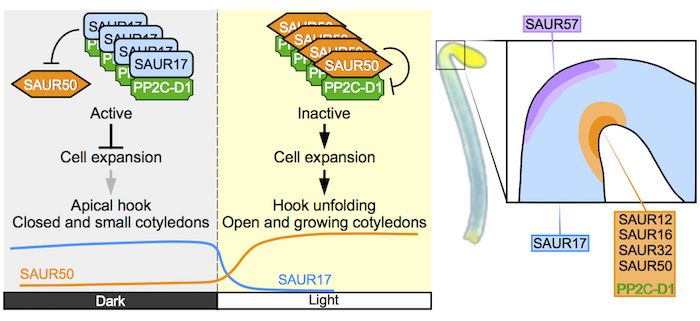
Background: Many land plants start their lifecycles in the dark as seeds that germinate in the soil. Under this condition, plants such as Arabidopsis thaliana become etiolated. The seedling’s two cotyledons (embryonic leaves) stay small and closed, and are folded under an apical hook, as the seedling pushes through the soil via its elongating hypocotyl (young stem). Once the seedling emerges and perceives light, the hook unfolds and the cotyledons open and start to enlarge and turn green. These dramatic changes in apical organ morphology are achieved through asymmetric cell growth, or differential cell-size control. SAUR (Small Auxin Up RNA) family proteins induce cell expansion by inhibiting the PP2C-D-depndent cell-size regulation system.
Question: We wanted to know whether SAUR17, the most strongly expressed SAUR gene in etiolated apical organs, plays a role in modulating the shape of the apical hook and cotyledons in the dark.
Findings: SAUR17 promotes apical hook development and closed cotyledons, whereas another group of SAURs including SAUR50 promotes hook and cotyledon opening. PP2C-D1 phosphatase, a known inhibitor of cell expansion, selectively associates with SAUR17 over other SAURs in etiolated seedlings to facilitate apical hook formation. While most SAURs including SAUR50 inhibit PP2C-D1 and induce cell expansion, SAUR17 does not inhibit PP2C-D1. Moreover, SAUR17 binds to PP2C-D1 with higher affinity, thereby protecting this phosphatase against SAUR50. This enables SAUR17 to keep PP2C-D1 active in suppressing cell enlargement in the dark. Upon the transition to light, SAUR17 is turned off and SAUR50 levels increase. This allows SAUR50 to bind to and inhibit PP2C-D1, causing the cells to expand. Because SAUR50 and PP2C-D1 are enriched in the inner side of the hook, the increased size of these cells leads to apical hook opening.
Next steps: Since SAUR17 is a non-inhibitory SAUR, comparative structure-function studies can be carried out to investigate how SAUR proteins inhibit PP2C-D1 activity at the molecular level. Also, hook and cotyledon morphology are determined by light and several phytohormones. Understanding how these signals are integrated to regulate aspects of the SAUR-PP2C-D1 cell-size control system will reveal how etiolation is sustained and terminated in plants such as Arabidopsis.