State Transition Regulation in Chlamydomonas
Ananya Mukherjee
University of Nebraska, Lincoln, Nebraska 68588
ORCID ID: 0000-0003-1802-1806
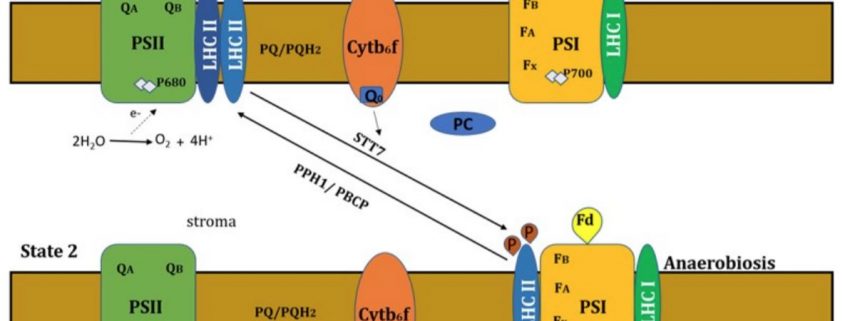
Photosynthetic organisms often face fluctuations in light quality and quantity. Green plants and algae have built-in mechanisms that allow them to adapt to such changes. One strategy involves modulating the activities of the two photosystems, PSI and PSII, through a reallocation of some of the light harvesting II (LHCII) complexes. This reallocation is called a state transition; State 1 (St 1) is when LHCII is associated solely with PSII, and St 2 is when some of the LHCII complexes are associated with PSI, directing more light to this photosystem. The transition between St 1 and St 2 is driven by the metabolic needs of the cell and the light environment.
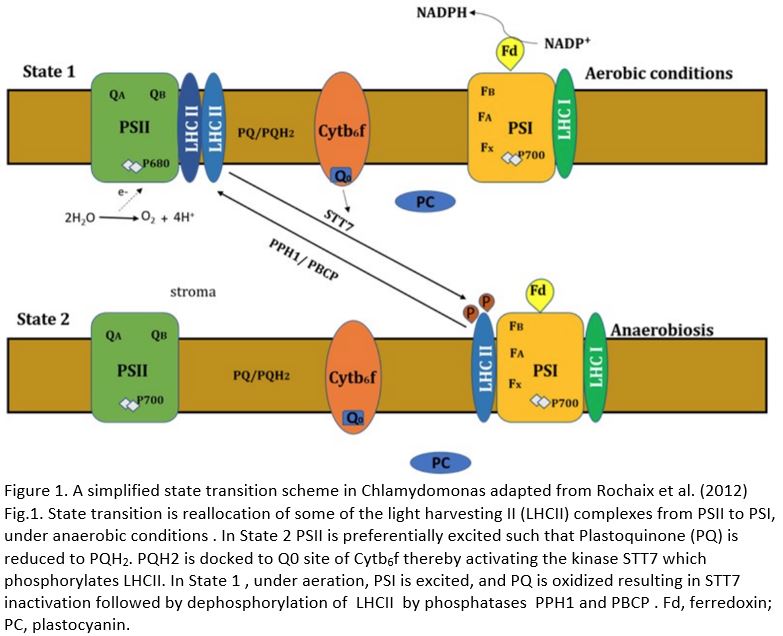
State transitions occur to fine-tune photosynthesis, balancing linear electron flow (LEF) and cyclic electron flow (CEF). Light captured by PSII initiates LEF by extracting electrons from water. These electrons are transferred through the plastoquinone (PQ) pool, the cytochrome b6f complex (Cytb6f), and plastocyanin to PSI. This electron transfer ultimately reduces ferredoxin (Fd) and NADP+ to NADPH and leads to the accumulation of protons in the lumen, creating a proton gradient that drives ATP synthesis. By contrast, light captured by PSI initiates CEF, which leads to proton gradient-driven ATP synthesis, but without the accompanying reduction of Fd and NADP+. The redox state of the plastoquinone (PQ) pool is a key factor controlling state transitions. A more reduced PQ pool favors St 2 and more light energy channeled to PSI, which has the effect of oxidizing the PQ pool.
Reorganization of LHCII as an acclimation to low light is mediated by phosphorylation of thylakoid proteins (Rochaix et al., 2012). In Arabidopsis thaliana, St 2 is favored by the phosphorylation of LHCII by the protein kinase STN7 (STATE TRANSITION 7) (Bellafiore et al., 2005). LHCII dephosphorylation by the protein phosphatase PPH1/TAP38 (PROTEIN PHOSPHATASE 1 / THYLAKOID ASSOCIATED PHOSPHATASE 38) favors St 1 (Pribil et al., 2010). STN7 has a paralog called STN8 (STATE TRANSITION 8), which phosphorylates thylakoid subunit proteins and is counteracted by PBCP (PHOTOSYSTEM II CORE PHOSPHATASE) (Puthiyaveetil et al., 2014).
Chlamydomonas acclimates to low light via a slightly different mechanism. St 2, which directs more light to PSI, is favored by anaerobiosis in the dark mainly because the altered metabolic demand during anoxia contributes to a more reduced PQ pool (Fig.1) (Bulté et al., 1990). In Chlamydomonas, STT7 is the ortholog of STN7, which phosphorylates LHCII trimer subunits (Depège et al., 2003). The ortholog of PPH1 in Chlamydomonas has not been characterized.
 In this issue of Plant Physiology, Cariti et al. (2020) identify the Chlamydomonas homologs of PPH1 and PBCP and characterize their roles in state transition.
In this issue of Plant Physiology, Cariti et al. (2020) identify the Chlamydomonas homologs of PPH1 and PBCP and characterize their roles in state transition.
To characterize the role of phosphatases in state transitions in Chlamydomonas, the authors identified Cre04.g218150 as the closest Chlamydomonas homolog of PPH1 (CrPPH1). The amino acid sequence of CrPPH1 has a 55% similarity and 36% identity to that of AtPPH1. They obtained a mutant line for the gene (pph1). Transition to St 2 is delayed, but still occurs, in the pph1 mutant, suggesting that another phosphatase may also contribute to state transition. In an effort to identify other phosphatases involved in state transitions, the authors used a genetic screen for mutants with impaired transitions from St 2 to St 1. Cre06.g2578850 (CrPBCP) was identified as the PBCP homolog in Chlamydomonas and showed increased phosphorylation of thylakoid proteins compared to the wild type, similar to its Arabidopsis homolog, although phosphoprotein analysis showed that CrPBCP has a different range of targets in Chlamydomonas than its homologue in Arabidopsis, where the major targets of PBCP are the subunits of the PSII core.
The mutant strains were crossed to generate a double mutant pph1;pbcp to test if the phosphatases had redundant function. State transition was monitored in pph1;pbcp and post aeration no St 1 transition was observed. This is indicative of the double mutant being locked into St 2. Thus, PPH1 and PBCP, unlike its Arabidopsis homolog, are both partially involved in the St 1 transition.
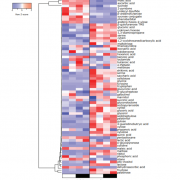
LHCII de-phosphorylation is an indicator of the St 2 to St 1 transition. In the pph1, pbcp, and pph1;pbcp mutants, an abundance of LHCII phosphorylation was observed during state transition, unlike in the wild type. To identify the target specificity of PPH1 and PBCP, Phos tag PAGE was carried out to assess the cumulative phosphorylation effect. Components of LHCII- LHCBM5, LHCB4, and LHCB5 are phosphorylated by both PPH1 and PBCP with PBPCP playing a more active role in the case of LHCB4. PPH1 dephosphorylates LHCBM1 and PBCP dephosphorylates LHCBM3 and LHCBM4/6/8. Additionally, it was observed that the PsbH subunit of PSII was dephosphorylated by PBCP and that PETO, a CEF regulatory protein (Takahashi et al., 2016), was dephosphorylated by both phosphatases.
Therefore, while some target proteins for the two phosphatases are different, there is some overlap as well. The organization of LHC subunits differs in Chlamydomonas and perhaps that is why the phosphatases PPH1 and PBCP play partially redundant roles in state transition. In future studies, it would be interesting to explore how the evolution of LHC in Chlamydomonas might have played a role in the divergent functions of these phosphatases.
Literature Cited
Bellafiore S, Barneche F, Peltier G, Rochaix J-D (2005) State transitions and light adaptation require chloroplast thylakoid protein kinase STN7. Nature 433: 892-895
Bulté L, Gans P, Rebéillé F, Wollman F-A (1990) ATP control on state transitions in vivo in Chlamydomonas reinhardtii. Biochimica et Biophysica Acta (BBA)-Bioenergetics 1020: 72-80
Depège N, Bellafiore S, Rochaix J-D (2003) Role of chloroplast protein kinase Stt7 in LHCII phosphorylation and state transition in Chlamydomonas. Science 299: 1572-1575
Pribil M, Pesaresi P, Hertle A, Barbato R, Leister D (2010) Role of plastid protein phosphatase TAP38 in LHCII dephosphorylation and thylakoid electron flow. PLoS biology 8
Puthiyaveetil S, Woodiwiss T, Knoerdel R, Zia A, Wood M, Hoehner R, Kirchhoff H (2014) Significance of the photosystem II core phosphatase PBCP for plant viability and protein repair in thylakoid membranes. Plant and Cell Physiology 55: 1245-1254
Rochaix J-D, Lemeille S, Shapiguzov A, Samol I, Fucile G, Willig A, Goldschmidt-Clermont M (2012) Protein kinases and phosphatases involved in the acclimation of the photosynthetic apparatus to a changing light environment. Philosophical Transactions of the Royal Society B: Biological Sciences 367: 3466-3474
Takahashi H, Schmollinger S, Lee J-H, Schroda M, Rappaport F, Wollman F-A, Vallon O (2016) PETO interacts with other effectors of cyclic electron flow in Chlamydomonas. Molecular plant 9: 558-568