A high-five for high light protection
Kasper van Gelderen
Utrecht University
[email protected]
Plants cannot live without light, but they also cannot live with too much light. Beyond a certain threshold, a high light intensity will damage the photosynthetic apparatus directly. Furthermore, high light leads to the production of reactive oxygen species that can cause widespread damage in the cell (Li et al., 2009). One way in which plants respond to high light stress is by converting some of the excess light into heat via non-photochemical quenching (NPQ) (Müller et al., 2001). Outdoors, light intensities can vary greatly in time and space: one part of the plant can experience high light stress, while another part is shaded. This shaded part of the plant might experience high light stress later on; therefore, it is advantageous for plants to have evolved a systemic signaling response to local high light stress that primes the whole plant to respond better to high light (Karpinski et al., 1999). Several ideas of how the plant transduces such a systemic signal have been postulated, such as a calcium or reactive oxygen species wave and ABA synthesis (Gilroy et al., 2016; Devireddy et al., 2018). However, no direct links between these signaling mechanisms and NPQ systemic high light acclimation have been identified.
 In this issue of Plant Physiology, Jiang et al. (2020) identify how the tomato plant (Solanum lycopersicum) systemically responds to high light and primes the NPQ mechanisms in parts of the plant that have not been exposed to high light. A light-induced transcription factor moves from one leaf to another to induce key enzymes in NPQ. Interestingly, this transcription factor, ELONGATED HYPOCOTYL5 (HY5), has come into the spotlight recently, because it is involved in systemic shoot–root signaling in response to shoot-perceived light (Chen et al., 2016; van Gelderen et al., 2018). In a recent study, the authors identified HY5 as a central component in the regulation of NPQ in tomato (Wang et al., 2018). This prompted Jiang et al. to investigate if HY5 could act as a systemic leaf-to-leaf signal in tomato.
In this issue of Plant Physiology, Jiang et al. (2020) identify how the tomato plant (Solanum lycopersicum) systemically responds to high light and primes the NPQ mechanisms in parts of the plant that have not been exposed to high light. A light-induced transcription factor moves from one leaf to another to induce key enzymes in NPQ. Interestingly, this transcription factor, ELONGATED HYPOCOTYL5 (HY5), has come into the spotlight recently, because it is involved in systemic shoot–root signaling in response to shoot-perceived light (Chen et al., 2016; van Gelderen et al., 2018). In a recent study, the authors identified HY5 as a central component in the regulation of NPQ in tomato (Wang et al., 2018). This prompted Jiang et al. to investigate if HY5 could act as a systemic leaf-to-leaf signal in tomato.
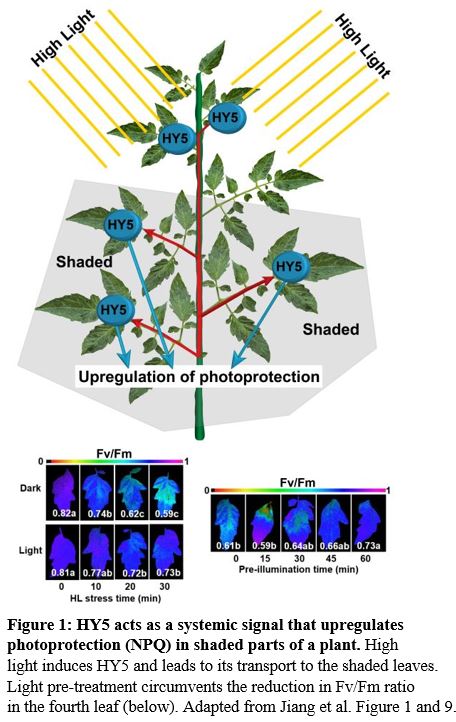
They exposed the upper part of the plant (5th–8th leaves) to high light. This pre-treatment primed the 4th leaf of the plant to respond better to the high light treatment later on (Figure 1). In the systemic 4th leaf of the plant, transcription and translation of NPQ enzymes were upregulated. The effect of high light stress and the light pre-treatment could be seen by observing the loss of photosynthesis efficiency via the Fv/Fm ratio (Figure 1, lower images). Additionally, HY5 transcription was upregulated in the fourth leaf, which already gives a hint to HY5 acting as a systemic signal, since HY5 can upregulate its own transcription (van Gelderen et al., 2018).
To tackle the question of whether HY5 itself could act as the systemic signal, the authors used a transgenic tomato line expressing tagged HY5 detectable by immunoblot. They grafted the upper part of this plant to the lower part of a non-transgenic wild-type (WT) plant, and then applied high light to the upper part. In this way the transgenic tagged HY5 could be detected in the WT plant. The experiment showed that HY5 could indeed move through the plant, but was it actually a systemic signal? To answer this question, the authors grafted the upper part of a hy5 RNAi knockdown line and the aforementioned HY5 overexpression line onto the lower parts of WT plants. They observed that the benefit of the light pre-treatment was lost on the hy5-rnai/WT grafted plant. However, in the HY5overexpression/WT plants, this benefit was enhanced compared to WT/WT grafted plants. In each case, the authors measured the effects using the photosynthetic efficiency and NPQ enzyme induction. Surprisingly, WT/hy5-rnai grafted plants, with the upper parts of WT plants grafted onto the lower parts of hy5-rnai plants, exhibited an induction of NPQ enzymes in the fourth systemic leaf. Although the induction was at a lower amount than in WT/WT plants, the result does show that a large part this systemic response can be attributed to HY5. Finally, the authors demonstrated that HY5 can bind directly to the regulatory regions of the genes encoding these essential NPQ enzymes.
In summary, the authors show that HY5 acts as a systemic, leaf-to-leaf signal in the high light acclimation response of tomato. Faster responses such as electrical signals or calcium waves may play an important role in this process, too, but the loss of the priming response evident in the grafting experiments argues that such rapid signaling is less important in this instance. The authors propose that it is beneficial to have a slower, longer lasting response to high light mediated by HY5 transport, in addition to a quick, but transient one. Such responses at different speeds would enable the plant to have adaptive flexibility when responding to an ever-changing light environment.
The study of Jiang et al. is the first to demonstrate a role for HY5 as a systemic signal between distal organs in the shoot. This finding has wide implications for systemic signaling in plant biology. Besides the HY5 transcription factor/regulator acting as a systemic signal, there is the well-known example of the FLOWERING TIME T (FT) transcription factor that moves from leaf to meristem and inflorescence, and more recently of another transcription factor, ELF4, that moves from shoot to root (Chen et al., 2020). Smaller secreted proteins like RALFs and CEPs can also move between shoot and root and vice versa. Many more similar transcriptional regulators could act as systemic signals. Their power lies in the fact that they can directly and broadly affect transcriptional networks. These mobile transcriptional regulators stretch the paradigms of plant signaling beyond hormones, electrical signals, calcium waves and small nucleotides. The sessile nature of plants keeps revealing interesting ways of signaling!
Literature Cited
Chen, W.W., Takahashi, N., Hirata, Y., Ronald, J., Porco, S., Davis, S.J., Nusinow, D.A., Kay, S.A., and Mas, P. (2020). A mobile ELF4 delivers circadian temperature information from shoots to roots. Nat. Plants 6: 416–426.
Chen, X., Yao, Q., Gao, X., Jiang, C., Harberd, N.P., Fu, X., Chen, X., Yao, Q., Gao, X., Jiang, C., Harberd, N.P., and Fu, X. (2016). Shoot-to-Root Mobile Transcription Factor HY5 Coordinates Plant Carbon and Nitrogen Acquisition Report Shoot-to-Root Mobile Transcription Factor HY5 Coordinates Plant Carbon and Nitrogen Acquisition. Curr. Biol.: 1–7.
Devireddy, A.R., Zandalinas, S.I., Gómez-Cadenas, A., Blumwald, E., and Mittler, R. (2018). Coordinating the overall stomatal response of plants: Rapid leaf-to-leaf communication during light stress. Sci. Signal. 11.
van Gelderen, K., Kang, C., Paalman, R., Keuskamp, D., Hayes, S., and Pierik, R. (2018). Far-Red Light Detection in the Shoot Regulates Lateral Root Development through the HY5 Transcription Factor. Plant Cell 30: 101–116.
Gilroy, S., Białasek, M., Suzuki, N., Górecka, M., Devireddy, A.R., Karpiński, S., and Mittler, R. (2016). ROS, calcium, and electric signals: Key mediators of rapid systemic signaling in plants. Plant Physiol. 171: 1606–1615.
Karpinski, S., Reynolds, H., Karpinska, B., Wingsle, G., Creissen, G., and Mullineaux, P. (1999). Systemic signaling and acclimation in response to excess excitation energy in Arabidopsis. Science (80-. ). 284: 654–657.
Li, Z., Wakao, S., Fischer, B.B., and Niyogi, K.K. (2009). Sensing and Responding to Excess Light. Annu. Rev. Plant Biol. 60: 239–260.
Müller, P., Li, X.P., and Niyogi, K.K. (2001). Non-photochemical quenching. A response to excess light energy. Plant Physiol. 125: 1558–1566.
Wang, F., Wu, N., Zhang, L., Ahammed, G.J., Chen, X., Xiang, X., Zhou, J., Xia, X., Shi, K., Yu, J., Foyer, C.H., and Zhou, Y. (2018). Light signaling-dependent regulation of photoinhibition and photoprotection in Tomato. Plant Physiol. 176: 1311–1326.