Small subunits can determine enzyme kinetics of tobacco Rubisco expressed in Escherichia coli (Nature Plants)

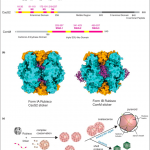
Rubisco is the enzyme responsible for the fixation of CO2 to ribulose-1,5-bisphosphate (RuBP) during photosynthetic reactions. However, this enzyme has some functional issues, such as a slow catalytic turnover rate and sensitivity to temperature and CO2, and it catalyzes a competing oxygenation process in C3 plants. Crops have reached a photosynthetic yield plateau that could be enhanced with a more efficient carbon fixation process. Plants have form-I Rubisco, with 8 large (RbcLs) and 8 small subunits (RbcSs). RbcLs tend to easily aggregate and depend on chaperones for proper folding and dimerization, impairing heterologous expression in bacteria for functional studies. Lin et al. modified and improved methods previously described to coexpress Rubisco with chaperonin and chaperones from Arabidopsis and tobacco using T7 promoters under autoinduction conditions. Eight different tobacco RbcSs were coexpressed with RbcL and assayed for proper assembly and kinetics efficiency. Rubisco assembled in E. coli from tobacco trichome RbcS-Tc showed higher turnover but a lower affinity for CO2 than native mesophyll RbcSs, as previously described for Rubisco found in trichomes. These results demonstrate that RbcSs can determine tobacco Rubisco kinetics when expressed in E. coli. The described strategies can be further used to screen and engineer Rubisco with improved function from other crop plants. (Summary by Camila Ribeiro @camicribeiro86) Nature Plants 10.1038/s41477-020-00761-5