Glucose-Induced Trophic Shift in an Endosymbiotic Dinoflagellate
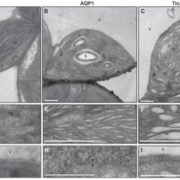
 Dinoflagellates in the genus Symbiodinium have the ability to enter into endosymbiotic associations with corals, providing the metabolic basis for the highly productive and biologically diverse coral-reef ecosystems, as well as with other cnidarians, including sea anemones and jellyfish. The Symbiodinium-coral association is highly susceptible to environmental perturbations such as high temperature that can result in the loss of the algae from coral tissue (‘coral bleaching’) and ultimately lead to the death of the host and destruction of the reef. This phenomenon is plaguing reef communities world-wide. Metabolite exchange is essential to the success of coral-dinoflagellate symbioses. The algal symbionts are photosynthetically competent, and much of the CO2 that they fix can be accessed by the host via translocation of photosynthetic products (e.g., sugars, amino acids, lipids, carbohydrates, and small peptides) from the algae to the host. Although carbon flow between the partners is a hallmark of this mutualism, the mechanisms governing this flow and its impact on symbiosis remain poorly understood. Symbiodinium strain SSB01 can grow photoautotrophically, or it can grow mixotrophically or heterotrophically when supplied with glucose, a metabolite normally transferred from the alga to its host. Xiang et al. (10.1104/pp.17.01572) now show that glucose supplementation of SSB01 cultures causes a loss of pigmentation and photosynthetic activity, disorganization of thylakoid membranes, accumulation of lipid bodies, and alterations of cell-surface morphology. Glucose-supplemented cells exhibited a marked reduction in levels of plastid transcripts encoding photosynthetic proteins, although most nuclear-encoded transcripts (including those for proteins involved in lipid synthesis and formation of the extracellular matrix) exhibited little change in their abundances (except for a doubling of some nuclear-encoded transcripts for sugar transporters). Thus, Symbiodinium undergoes dramatic physiological changes in response to glucose that are not reflected by major changes in the abundances of nuclear-encoded transcripts and thus presumably reflect post-transcriptional regulatory processes.
Dinoflagellates in the genus Symbiodinium have the ability to enter into endosymbiotic associations with corals, providing the metabolic basis for the highly productive and biologically diverse coral-reef ecosystems, as well as with other cnidarians, including sea anemones and jellyfish. The Symbiodinium-coral association is highly susceptible to environmental perturbations such as high temperature that can result in the loss of the algae from coral tissue (‘coral bleaching’) and ultimately lead to the death of the host and destruction of the reef. This phenomenon is plaguing reef communities world-wide. Metabolite exchange is essential to the success of coral-dinoflagellate symbioses. The algal symbionts are photosynthetically competent, and much of the CO2 that they fix can be accessed by the host via translocation of photosynthetic products (e.g., sugars, amino acids, lipids, carbohydrates, and small peptides) from the algae to the host. Although carbon flow between the partners is a hallmark of this mutualism, the mechanisms governing this flow and its impact on symbiosis remain poorly understood. Symbiodinium strain SSB01 can grow photoautotrophically, or it can grow mixotrophically or heterotrophically when supplied with glucose, a metabolite normally transferred from the alga to its host. Xiang et al. (10.1104/pp.17.01572) now show that glucose supplementation of SSB01 cultures causes a loss of pigmentation and photosynthetic activity, disorganization of thylakoid membranes, accumulation of lipid bodies, and alterations of cell-surface morphology. Glucose-supplemented cells exhibited a marked reduction in levels of plastid transcripts encoding photosynthetic proteins, although most nuclear-encoded transcripts (including those for proteins involved in lipid synthesis and formation of the extracellular matrix) exhibited little change in their abundances (except for a doubling of some nuclear-encoded transcripts for sugar transporters). Thus, Symbiodinium undergoes dramatic physiological changes in response to glucose that are not reflected by major changes in the abundances of nuclear-encoded transcripts and thus presumably reflect post-transcriptional regulatory processes.