Shaping the Plant Cell Wall: Molecular Characteristics of XOAT1 in Polysaccharide Acetylation
Plant cell walls provide mechanical support to plant cells, determine their size and shape, and influence plant development and stress responses. The plant cell wall is composed mainly of polysaccharides, including cellulose, hemicellulose and pectins, with smaller amounts of phenolic polymers and proteins embedded in the polysaccharide matrix. Many of these polysaccharides are O-acetylated, which defines the architecture and mechanical strength of the plant cell wall by dictating whether two molecules can become crosslinked (Pauly and Ramírez, 2018). Despite the significance of polysaccharide O-acetylation in plant cell wall development, little is known about the detailed mechanisms underlying this process.
 O-acetylation of xylan, a major type of hemicellulose in the plant cell wall, influences the interaction between xylan and other cell wall polymers, and therefore shapes the cell wall (Grantham et al., 2017). Previous studies had identified the Arabidopsis (Arabidopsis thaliana) xylan specific O-acetyltransferase (XOAT1) for O-acetylation at the 2-position of xylosyl backbone residues (Grantham et al., 2017; Urbanowicz et al. 2014; Xiong et al. 2013). Building on these initial findings, Lunin et al. (2020) now present structural and mechanistic details of XOAT1 that advance our understanding of the molecular mechanism of polysaccharide acetylation in plant cell walls.
O-acetylation of xylan, a major type of hemicellulose in the plant cell wall, influences the interaction between xylan and other cell wall polymers, and therefore shapes the cell wall (Grantham et al., 2017). Previous studies had identified the Arabidopsis (Arabidopsis thaliana) xylan specific O-acetyltransferase (XOAT1) for O-acetylation at the 2-position of xylosyl backbone residues (Grantham et al., 2017; Urbanowicz et al. 2014; Xiong et al. 2013). Building on these initial findings, Lunin et al. (2020) now present structural and mechanistic details of XOAT1 that advance our understanding of the molecular mechanism of polysaccharide acetylation in plant cell walls.
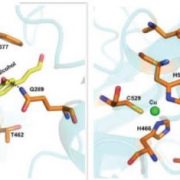
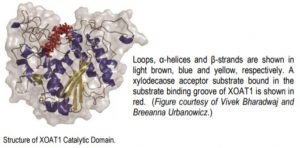
The authors first determined the structure of the catalytic domain of XOAT1: it consists of two unequal lobes separated by a deep cleft with a Ser–His–Asp catalytic triad located at the bottom of the cleft (see Figure). The overall conformation of this catalytic domain is distinct from any structures deposited in the Protein Data Bank, but the α/β/α arrangement of the larger lobe shares some similarities with members of the GDSL-like lipase/acylhydrolase family.
To address the catalytic mechanism of XOAT1, the authors then determined whether XOAT1 showed any site preference along the xylan backbone by continuously monitoring the products released by the enzyme. XOAT1 specifically transferred O-acetyl moieties solely to the 2-position of the xylan backbone. However, longer incubation times saw the spontaneous migration to the 3-position in the absence of XOAT1, which is therefore not enzyme-driven. Next, the authors asked whether an acetyl-enzyme intermediate formed during XOAT1 catalysis. Toward this end, they incubated XOAT1 with acetyl donors in the absence of acetyl acceptors, digested the enzyme with trypsin, and analyzed the resulting peptides by mass spectrometry. Their results confirmed the formation of an acetyl-enzyme intermediate within the catalytic triad, suggesting a double-displacement mechanism for the O-acetylation reaction catalyzed by XOAT1. Finally, mutagenic analyses and molecular simulations substantiated the indispensable role of the catalytic triad and revealed the ability of the cleft on the surface of the protein to act as a substrate binding groove to stabilize acceptor and donor substrates. The authors conclude that XOAT1 catalyzes xylan 2-O-acetylation through a double displacement bi-bi reaction involving the formation of an acetyl-enzyme intermediate.
The structural and functional characteristics of enzymes responsible for polysaccharide modifications are essential for understanding how plants build their cell walls to accommodate growth patterns. Thus, the structural information and working mechanisms of XOAT1, revealed in this study, represent a major step towards deciphering the mechanisms involved in shaping the plant cell wall. It also sets the stage for future research to explore how to produce plant biomass more amenable for enzymatic digestion and release of these trapped sugars.
Yingqi Cai
Biology Department, Brookhaven National Laboratory
Upton, New York
ORCID: 0000-0002-0357-5809
REFERENCES
Grantham, N.J., Wurman-Rodrich, J., Terrett, O.M., Lyczakowski, J.J., Stott, K., Iuga, D., Simmons, T.J., Durand-Tardif, M., Brown, S.P., Dupree, R., Busse-Wicher, M., and Dupree, P. (2017). An even pattern of xylan substitution is critical for interaction with cellulose in plant cell walls. Nat. Plants 3: 859-865.
Pauly, M., and Ramírez, V. (2018). New insights into wall polysaccharide O-acetylation. Front. Plant Sci. 9: 1210.
Urbanowicz, B.R., Peña, M.J., Moniz, H.A., Moremen, K.W., and York, W.S. (2014). Two Arabidopsis proteins synthesize acetylated xylan in vitro. Plant J. 80: 197-206.
Xiong, G., Cheng, K., and Pauly, M. (2013). Xylan O-acetylation impacts xylem development and enzymatic recalcitrance as indicated by the Arabidopsis mutant tbl29. Mol. Plant 6: 1373-1375.