A New Polysaccharide with a Long Evolutionary History
By
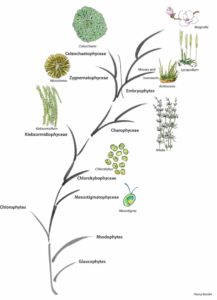
Evolution of the green plant lineage. (Figure courtesy of Panny Kondor.)
For the first time in a very long time, a new polysaccharide is reported in plants. Roberts et al. (2018) discovered an arabinoglucan in the moss Physcomitrella patens. This discovery came about not as a result of biochemical characterization of the moss cell wall, but rather as a targeted study of the gene Pp3c12_24670 by transient expression analysis in Nicotiana benthamiana and reduced or enhanced expression in the moss, identifying not only the polymer, but also its synthase. The gene product includes the cellulose synthase superfamily signature sequence D,D,D,QxxRW, which was discovered through a transcriptomic approach by Delmer and colleagues in 1996 after years of struggle with biochemical methods (Pear et al., 1996).
Cellulose synthase has a prokaryotic origin and the arabinoglucan synthase, AGlcS, is strikingly similar to the prokaryotic homologs as well as cellulose synthases in fungi, oomycetes, and even a few animals. While some are organized as linear terminal complexes in the plasma membrane and produce cellulose, others, including AGlcS, have been neofunctionalized to synthesize polysaccharides different from cellulose. The arabinoglucan is a mixed-linkage glucan in which glucose molecules are arranged in β-1,4 linkages, as in cellulose, while the arabinosyl residues are β-1,3-linked in-chain with the glucan. The work by Roberts et al. leaves little doubt that AGlcS alone is capable of synthesizing a polymer with interspersed 1,3-linked arabinosyl residues.
Neofunctionalization of a member of the plant cellulose synthase superfamily to produce a mixed-linkage polysaccharide has occurred more than once. The late Bruce Stone and his fellow Australian colleagues who co-authored the AGlcS study proved first that a barley (Hordeum vulgare) member of family CslF and subsequently CslH of the cellulose synthase superfamily produce β-(1,4;1,3) glucan when expressed in Arabidopsis thaliana, and later demonstrated that subtle changes to sequences near the active site of CslF modulate the interspersion of β-1,3 linkages in the polysaccharide (Dimitroff et al., 2016). Even though the shift from a homopolymer synthase to a heteropolymer synthase appears evolutionarily simple, we are nowhere near a mechanistic understanding of how a glycosyltransferase (GT) with a single active site can do this, and whether a given enzyme will produce a homopolymer cannot be inferred from the sequence alone.
Roberts et al. propose that the AGlcS ortholog in spikemoss (Selaginella) most likely produces a β-(1,4;1,3) glucan akin to the polymer known from horsetails and grasses. Horsetails (Equisetum) emerge later in the fossil record than spikemoss (both seedless plants) and horsetails also have an AGlcS ortholog, which they may use to produce a mixed-linkage glucan rather than using a GT belonging to any of the cellulose synthase superfamily clades that presently are considered grass-specific (CslF, CslH, and CslJ).
Neofunctionalization could have occurred when the modern rosette-shaped cellulose synthase complex evolved near the base of the charophytic algae, making linear terminal complex cellulose synthases redundant. This is in line with the observation that the occurrence of rosette-forming cellulose synthases coincided with or led to an evolutionary hotspot, resulting in the modern plant cell wall. Klebsormidium is the deepest branching extant representative featuring distinctly advanced plant cell wall features (Jensen et al., 2018), whereas even deeper branching charophytes (represented by Mesostigma in the figure) sport a distinctly different cell wall. An unbroken line of heritage of the Physcomitrella AGlcS from the last common ancestor of mosses among the Charophytes and then evolutionarily deeper from the common ancestor of chlorophytes and rhodophytes, which had linear terminal complex cellulose synthases, and even further back towards the origin of Eukaryotes, seems
likely. Horizontal gene transfer might help explain the occurrence in e.g., Dictyostelium and stramenopiles, possibly at the time of the secondary endosymbiosis that transferred the rhodophyte chloroplast into stramenopiles. While other scenarios should be entertained, this interpretation appears to be parsimonious, and it highlights the importance of the present work in furthering our understanding of early plant cell wall evolution.
The discovery of the mixed linkage arabinoglucan presents us with the first known polysaccharide synthesized by a member of the cellulose synthase superfamily featuring both hexosyl and pentosyl residues in its backbone. This reveals great flexibility in the gene family and illustrates how extant plant sequences that feature pronounced similarities to the prokaryotic origins should not be viewed as necessarily “primordial” or living fossils, as they have continued to evolve since divergence and may have undergone neofunctionalization. Great care should be exercised when inferring function from structure. The work by Roberts and colleagues opens a number of lines of scientific enquiry that may shed light on the earlier phases of plant cell wall evolution and may provide clues to inferring function from sequence in the cellulose synthase superfamily.
REFERENCES
Dimitroff, G., Little, A., Lahnstein, J., Schwerdt, J.G., Srivastava, V., Bulone, V., Burton, R.A., and Fincher, G.B. (2016). (1,3;1,4)-beta-Glucan biosynthesis by the CSLF6 enzyme: Position and flexibility of catalytic residues influence product fine structure. Biochemistry 55: 2054-2061.
Jensen, J.K., et al. (2018) Identification of an algal xylan synthase indicates that there is functional orthology between algal and plant cell wall biosynthesis. New Phytol. 218: 1049-1060.
Pear, J.R., Kawagoe, Y., Schreckengost, W.E., Delmer D.P., and Stalker, D.M. (1996) Higher plants contain homologs of the bacterial celA genes encoding the catalytic subunit of cellulose synthase. PNAS 93: 12637-12642.
Roberts, A.W., et al. (2018). Functional Characterization of a Glycosyltransferase from the Moss Physcomitrella patens Involved in the Biosynthesis of a Novel Cell Wall Arabinoglucan. Plant Cell 30: doi:10.1015/tpc18.0082.