The Lure of Lignin: Deciphering High-value Lignin Formation in Seed Coats
Lignin is a major functional component of plant secondary cell walls and the second most abundant biological polymer on Earth after cellulose. Understanding the biosynthesis of lignin has huge practical implications because lignin is a major by-product of processes that use cellulosic biomass for industrial purposes, such as bioethanol production. To increase the overall efficiency and value of cellulose processing, it is desirable to enhance the usability of the lignin by-product by optimizing its composition in source material.
It was long thought that lignin was derived from the polymerization of combinations of three monolignols: p-coumaryl alcohol, sinapyl alcohol, or coniferyl alcohols. However, when examining the composition of the seed coats of several monocots and dicots, researchers discovered a unique type of naturally occurring lignin, catechyl lignin (C-lignin) formed from the polymerization of caffeyl-alcohol (Chen et al., 2012). The modified chemical structure of C-lignin has shown promise towards enhanced recovery and downstream usage of lignin under biorefinery conditions (Li et al., 2018). Therefore, the development of biocellulose feedstocks with a high C-lignin content is a current biotechnology goal.
During lignin formation, monolignols are exported to the apoplast where they undergo enzyme-catalyzed oxidation followed by spontaneous free radicle-based polymerization into lignin. Apoplastic peroxidases and laccases are thought to be responsible for catalyzing the oxidation step; however, their large gene families and general promiscuity towards substrates have impeded our understanding of their individual roles in determining lignin composition. The control of lignin composition is thought to lie solely with the relative availability of exported monolignols because no laccase or peroxidase has been demonstrated to discriminate effectively between monolignol substrates.
 During seed development in the model plant Cleome hassleriana, lignin biosynthesis in the seed coat switches from coniferyl-based G-lignin to caffeyl-based C-lignin at approximately 12 days after pollination. The highly restricted occurrence of C-lignin to specific stages of seed coat development makes it an important target for investigations into how lignin composition specificity is determined. Previous work established that accumulation of the caffeyl alcohol monomers is due to the temporal expression of upstream biosynthetic enzymes (Zhuo et al., 2019). Here, in their recent Plant Cell paper, Xin Wang et al. (2020) sought to determine whether the expression of specific lignin polymerizing enzymes, namely laccases and peroxidases, is also required for C-lignin formation.
During seed development in the model plant Cleome hassleriana, lignin biosynthesis in the seed coat switches from coniferyl-based G-lignin to caffeyl-based C-lignin at approximately 12 days after pollination. The highly restricted occurrence of C-lignin to specific stages of seed coat development makes it an important target for investigations into how lignin composition specificity is determined. Previous work established that accumulation of the caffeyl alcohol monomers is due to the temporal expression of upstream biosynthetic enzymes (Zhuo et al., 2019). Here, in their recent Plant Cell paper, Xin Wang et al. (2020) sought to determine whether the expression of specific lignin polymerizing enzymes, namely laccases and peroxidases, is also required for C-lignin formation.
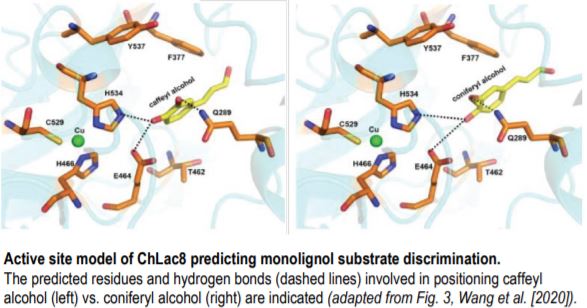
Examination of transcriptomic data from various Cleome tissues indicated a strong ontogenic correlation between the expression of the laccase gene ChLAC8 and C-lignin formation. Enzymatic analysis of heterologously expressed ChLAC8 revealed clear monolignol substrate specificity consistent with a role in C-lignin formation. That is, ChLAC8 catalyzed caffeyl-alcohol polymerization but not coniferyl-alcohol polymerization. A molecular basis for this substrate discrimination was presented following a series of molecular docking experiments (see Figure). Therefore, it appeared that both the accumulation of caffeyl-alcohol monomers and the expression of ChLAC8 were required for the formation of C-lignin within seed coats.
The authors then pursued experiments with multiple transgenic lines to confirm that these specific conditions (caffeyl-alcohol supply and ChLAC8 expression) in Arabidopsis thaliana inflorescence stems or Medicago truncatula hairy root cultures are sufficient for the exogenous formation of the atypical C-lignin, albeit at low levels. This result establishes an important precedent for modifying lignin composition in vivo. Continued metabolic engineering efforts are now needed to develop tissues capable of producing C-lignin at an industrial scale and unlocking the potential of its desirable chemical makeup.
Brendan M O’Leary
ARC Centre of Excellence in Plant Energy Biology
University of Western Australia
ORCID: 0000-0002-8770-155X
REFERENCES
Chen, F., Tobimatsu, Y., Havkin-Frenkel, D., Dixon, R.A., and Ralph, J. (2012). A polymer of caffeyl alcohol in plant seeds. Proc Natl Acad Sci U S A 109: 1772-1777.
Li, Y., Shuai, L., Kim, H., Motagamwala, A.H., Mobley, J.K., Yue, F., Tobimatsu, Y., Havkin-Frenkel, D., Chen, F., Dixon, R.A., Luterbacher, J.S., Dumesic, J.A., and Ralph, J. (2018). An “ideal lignin” facilitates full biomass utilization. Sci Adv 4: eaau2968.
Zhuo, C., Rao, X., Azad, R., Pandey, R., Xiao, X., Harkelroad, A., Wang, X., Chen, F., and Dixon, R.A. (2019). Enzymatic basis for C-lignin monomer biosynthesis in the seed coat of Cleome hassleriana. Plant J. 99: 506-520.