Life of PPi: Soluble PPases and H+-PPase act cooperatively to keep pyrophosphate levels in check
Inorganic phosphate (PPi) is a byproduct of many metabolic reactions, including those involved in sucrose, sugar nucleotide, and cellulose biosynthesis. Although PPi is an important phosphate donor and source of cellular energy, high levels of cytosolic PPi are toxic, disrupting the metabolic reactions that produce it. Thus, maintaining low cytosolic PPi levels, through PPi hydrolysis, is critical.
In most organisms, the cytosolic soluble pyrophosphatases (sPPases) are solely responsible for catalyzing PPi hydrolysis. Cytosolic sPPases are present in plants too; the Arabidopsis thaliana genome encodes five isoforms, PPa1–5 (Gutiérrez-Luna, 2016). However, a proton-pumping pyrophosphatase localized to the tonoplast, H+-PPase (encoded by AtVHP1/FUGU5), has been proposed to be a master regulator of cytosolic PPi homeostasis in plant cells that recycles the PPi generated during processes such as cellulose biosynthesis (Ferjani et al., 2011), calling into question the role of cytosolic sPPases in PPi homeostasis in plants.
Now, Segami et al. (2018) have addressed this question using a series of mutants lacking individual PPa isoforms, and higher-order mutants lacking four of the isoforms (ppa1-1 ppa2-1 ppa4-1 ppa5-1) and one of the isoforms plus H+-PPase (e.g., fugu5-1 ppa1-1). GFP fusion analysis revealed that PPa isoforms were localized to the cytosol and nucleus. Whereas PPA1, 2, 4, and 5 were widely distributed throughout the plant, PPA3 was confined to pollen and root hair cells. Heterologous expression of the Arabidopsis PPA isoforms in a yeast mutant lacking soluble PPase showed that each of the Arabidopsis isoforms had pyrophosphatase activity.
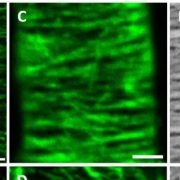
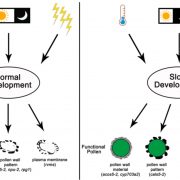
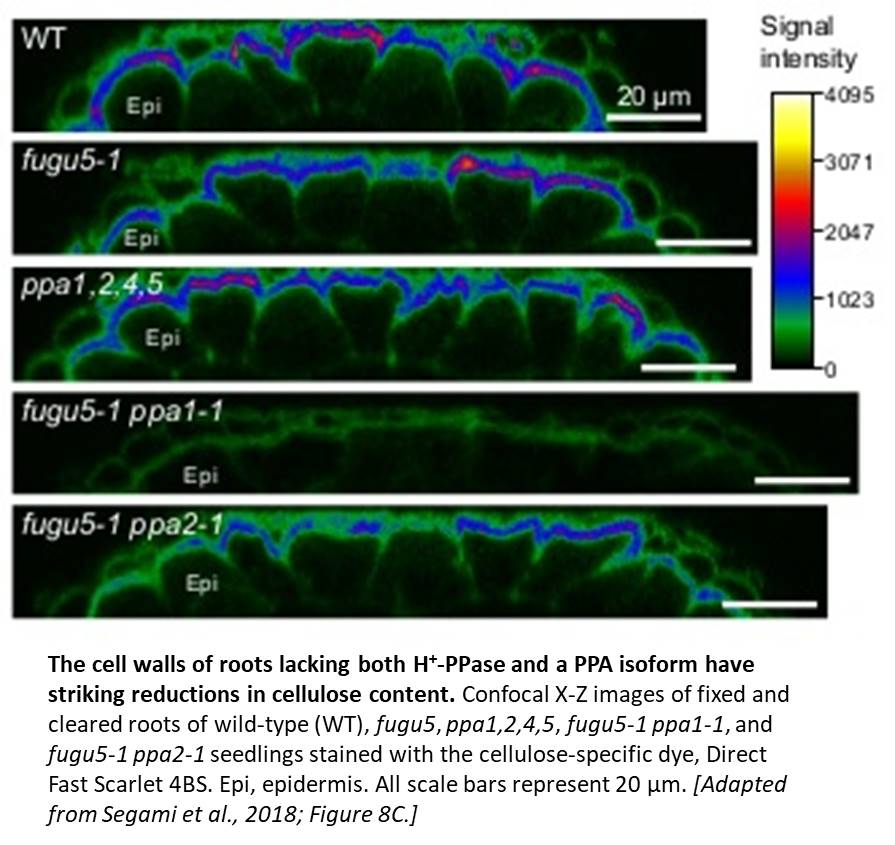
Although most of the mutants appeared normal, the authors noticed that some of the double knockout mutants, particularly fugu5-1 ppa1-1, had defects, including reduced hypocotyl and root elongation, aberrant cell wall morphology, and atrophied rosette leaves. Closer inspection showed that the double mutants had striking increases in PPi content; fugu5-1 ppa1-1 plants had higher levels of PPi than fugu5-1, even though ppa1-1 plants had PPi levels that were comparable to those of the wild type. Furthermore, the authors detected defects in cell wall composition in fugu5-1 ppa1-1 roots, including reduced levels of cellulose (see figure) and callose and changes in monosaccharide content. These differences weakened the cell walls of the double mutant, rendering them more likely to burst following small changes in osmotic pressure. Moreover, the fugu5-1 ppa1-1 mutant had higher levels of starch than the wild type and ectopically accumulated starch grains in root and hypocotyl cells, indicating a shift in metabolism from cell proliferation and elongation to starch biosynthesis.
Together, these findings highlight the importance of PPi turnover for normal plant growth and development and reveal that soluble PPases cooperate with H+-PPase to maintain PPi homeostasis in the cytosol of plant cells.
References
Ferjani, A., Segami, S., Horiguchi, G., Muto, Y., Maeshima, M., and Tsukaya, H. (2011). Keep an eye on PPi: the vacuolar-type H+-pyrophosphatase regulates postgerminative development in Arabidopsis. Plant Cell 23: 2895–2908.
Gutiérrez-Luna, F.M., De La Sancha, E.N., Valencia-Turcotte, L.G., Vázquez-Santana, S., and Rodríguez-Sotres, R. (2016). Evidence for a non-overlapping subcellular localization of the family I isoforms of soluble inorganic pyrophosphatase in Arabidopsis thaliana. Plant Sci. 253: 229–242.
Segami, S., Tomoyama, T., Sakamoto, S., Gunji, S., Fukuda, M., Kinoshita,S., Mitsuda, N., Ferjani, A., and Maeshima, M.. Plant Cell: doi.org/10.1105/tpc.17.00911.