Live and Let Die: Phosphatidic Acid Modulates the Self-Incompatibility Response
Pollen tubes are remarkable vehicles that deliver immobile sperm nuclei from the stigma to the ovule during angiosperm reproduction. Their journey delicately balances turgor pressure with the precise spatiotemporal regulation of polarized growth machinery to navigate pollen tubes and their cargo to the female gametophyte. To optimize genetic diversity, nearly half of all flowering plant species have developed a strategy known as self-incompatibility (SI) that selectively arrests growth of closely-related (incompatible) pollen tubes while allowing passage of unrelated (compatible) donors (Fujii et al., 2016). In this issue, Chen and colleagues (Chen et al., 2018) identify a protective mechanism that uses phosphatidic acid (PA) to delay the effects of SI cytotoxicity.
One of the best understood SI mechanisms uses a maternally-expressed S-locus RNase (S-RNase) to trigger RNA degradation and programmed cell death in incompatible pollen tubes while leaving compatible pollen tubes unharmed (McClure et al., 2011). Selectivity is achieved by a second S-locus allele expressed in pollen that encodes an F-box ubiquitin E3 ligase capable of initiating S-RNase degradation in a haplotype-dependent manner. Compatible pollen tubes evade death by destroying the S-RNase, whereas the inability to clear S-RNases is fatal for incompatible donors.
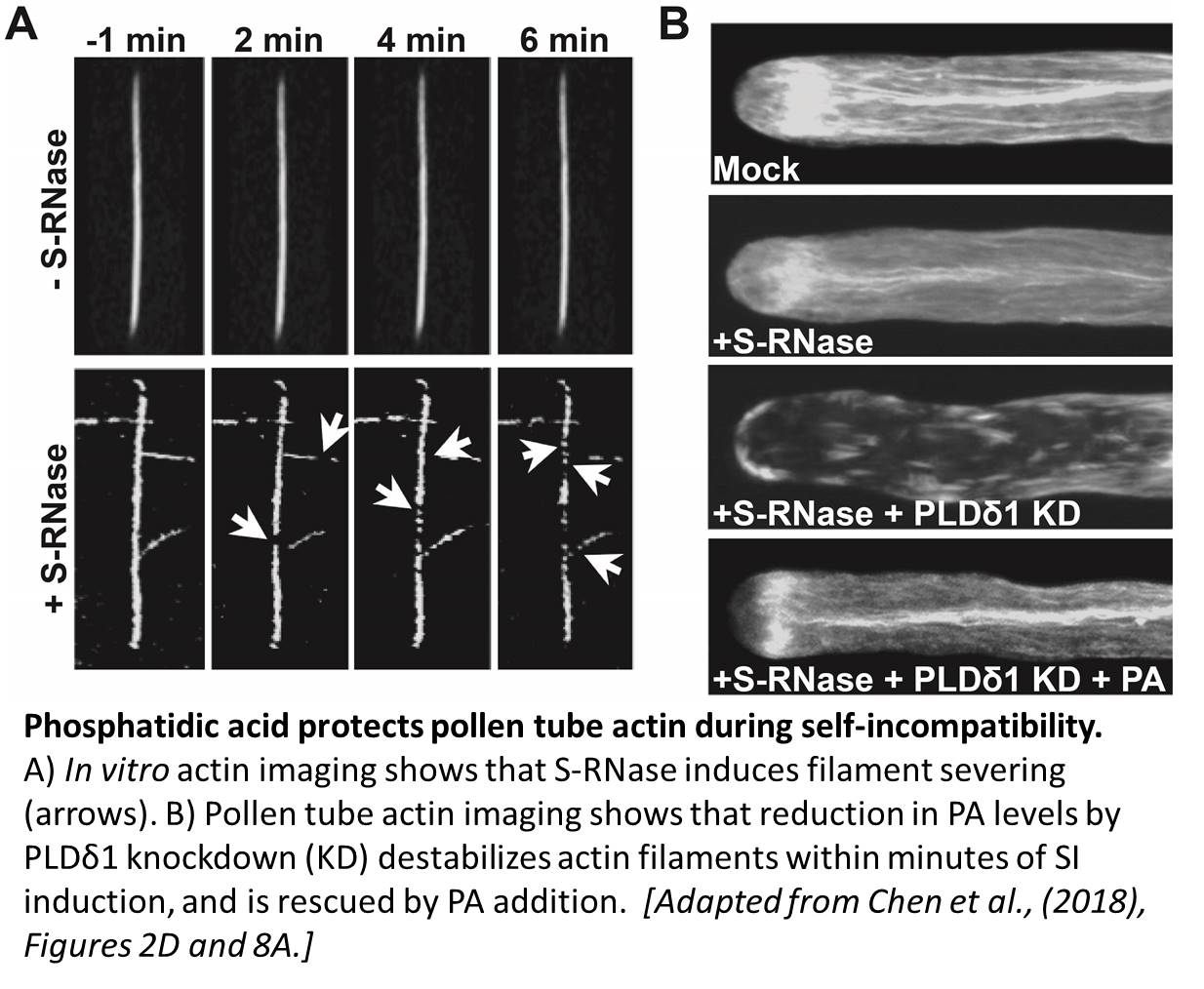
While RNA degradation has a well-established role in SI, the authors report that Pyrus bretschneideri (pear) S-RNases also have actin filament binding and severing activities that promote SI cytotoxicity (Chen et al., 2018). This finding links S-RNases to an actin destabilization phenomenon widely-observed in SI pollen tubes. Given the exquisite sensitivity of polarized tip-growing cells to actin perturbations, the actin-severing activity of S-RNase offers a mechanism to quickly sabotage SI pollen tube growth and induce cell death.
In addition, the authors discover a specific phospholipase D variant that dramatically elevates PA levels within minutes of SI elicitation. Whereas the rapid kinetics suggest that this PA spike could be a driver of cytotoxicity, an array of knockdown, pharmacological, and complementation experiments instead show that PA dampens the SI response. The protective properties of PA stem from its ability to stabilize the actin cytoskeleton from disassembly. One possibility is that PA directly inhibits S-RNase actin-severing activity, however the authors exclude this scenario with biochemical experiments. Thus, the precise mechanism by which PA initially protects pollen tubes from SI cytotoxicity remains an open question.
Importantly, these findings provide evidence for the “S-RNase threshold phenomenon” hypothesis which postulates that a sustained minimum level of S-RNase activity is necessary to fully activate the SI response (Soulard et al., 2014). Why is this protective mechanism necessary at all? Perhaps PA tunes this threshold to prevent inadvertent SI induction in compatible pollen tubes, thereby offering an escape from programmed cell death. Whatever the reason, the findings in this report suggest that the SI system acts as a gatekeeper that makes more nuanced life-or-death decisions than previously appreciated.
REFERENCES
Chen, J., Wang, P., de Graaf, B.H.J., Zhang, H., Jiao, H., Tang, C., Zhang, S., and Wu, J. (2018). Phosphatidic acid mitigates S-RNase signaling in pollen by stabilizing the actin cytoskeleton. The Plant Cell. Published April 2018,DOI: https://doi.org/10.1105/tpc.18.00021
Fujii, S., Kubo, K.-i., Takayama, S. (2016). Non-self- and self-recognition models in plant self-incompatibility. Nat. Plants. 2: 16130.
McClure, B., Cruz-García, F., and Romero C. (2011). Compatibility and incompatibility in S-RNase-based systems. Ann. Bot. 108: 647-658.
Soulard, J., Bolvin, N., Morse, D., Cappadocia, M. (2014). eEF1A is an S-RNase binding factor in self-incompatible Solanum chacoense. PLoS One 9: e90206.