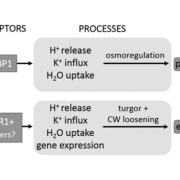
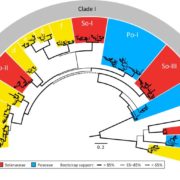
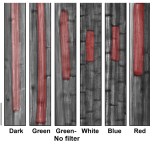
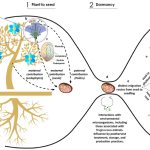
Insights into the chloroplast division site regulators and light
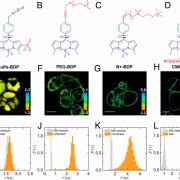
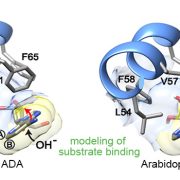
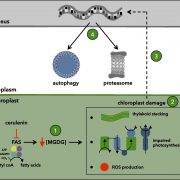
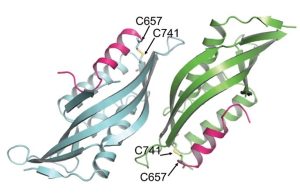 Chloroplasts divide by binary fission driven by a protein ring, the position of which is regulated by the Min system (derived from the system in bacteria). The inner envelope membrane protein PARC6 (PARALOG OF ARC6) is a key component. Here Sun et al. generated crystal structures showing that PARC6 interacts with the outer envelope membrane protein PDV1 (PLASTID DIVISION1). There are two interaction sites, one inside a pocket-like structure of PARC6 and one in a hydrophobic region on the lid of PARC6. Interaction at both sites leads to PARC6 dimerization, which is needed for correct protein function and chloroplast division. This PARC6-PDV1 interaction is redox regulated. Under oxidizing conditions, a disulfide bond within PARC6 blocks the binding pocket and inhibits the PDV1 interaction. In vitro, the reducing agent DTT reduces this bond to permit the PARC6-PDV1 interaction. In planta, this same reaction is triggered upon transition to light, driven by the accumulation of reductants. Therefore, this dynamic PARC6-PDV1 interaction ensures that chloroplast division is coordinated with light. (Summary by Rose McNelly @RoseMcN) Proc. Natl. Acad. Sci. USA 10.1073/pnas.2215575120
Chloroplasts divide by binary fission driven by a protein ring, the position of which is regulated by the Min system (derived from the system in bacteria). The inner envelope membrane protein PARC6 (PARALOG OF ARC6) is a key component. Here Sun et al. generated crystal structures showing that PARC6 interacts with the outer envelope membrane protein PDV1 (PLASTID DIVISION1). There are two interaction sites, one inside a pocket-like structure of PARC6 and one in a hydrophobic region on the lid of PARC6. Interaction at both sites leads to PARC6 dimerization, which is needed for correct protein function and chloroplast division. This PARC6-PDV1 interaction is redox regulated. Under oxidizing conditions, a disulfide bond within PARC6 blocks the binding pocket and inhibits the PDV1 interaction. In vitro, the reducing agent DTT reduces this bond to permit the PARC6-PDV1 interaction. In planta, this same reaction is triggered upon transition to light, driven by the accumulation of reductants. Therefore, this dynamic PARC6-PDV1 interaction ensures that chloroplast division is coordinated with light. (Summary by Rose McNelly @RoseMcN) Proc. Natl. Acad. Sci. USA 10.1073/pnas.2215575120