Extrapolating physiological response to drought through step-by-step analysis of water potential
Guillaume Charrier
Université Clermont Auvergne, INRAE, PIAF, F-63000 Clermont-Ferrand, France.
Water potential (Ψ) defines the energy required to move water between the different compartments of a closed system. Water flows passively in the direction of decreasing Ψ: in plants, from slightly negative Ψ at the soil and root level to strongly negative Ψ at the leaf level. The water deficit of the atmosphere is the driving force of water flow through the change in physical state (from liquid to vapor) that occurs at the leaf level. Such an atmospheric pump allows water to circulate along the soil–plant–atmosphere continuum, from the soil to the leaves and, ultimately, to the stomatal chamber through the xylem conducting elements. The upward movement of water via the roots, trunk, branches, and leaves is controlled by two complementary phenomena: cohesion and tension (Dixon & Joly, 1894). The vaporization of water in the stomata creates a negative hydrostatic pressure (or tension) that propagates along the water column due to the cohesion between water molecules (mainly due to hydrogen bonds). As the amount of water supplied by the root system and transported through the xylem cannot compensate for the transpired water, daily patterns in Ψleaf are observed, with minimum values when transpiration is at its maximum level (12:00–14:00 solar time, i.e., Ψmidday). At nighttime, transpiration ceases, the water diffuses passively along the water column, and the gradient of Ψ disappears. Ψpredawn thus represents the water potential of the soil prospected by the root system (Améglio et al., 1999).
The difference between Ψpredawn and Ψmidday is related to the regulation of plant water status in response to soil water content and, thus, to Ψpredawn. The correlation between Ψpredawn and Ψmidday has been used to describe the stringency of water regulation, notably through stomatal opening, root, stem, and leaf hydraulic conductances, and stem capacitance (Martinez-Vilalta et al., 2014). The slope of the linear regression between Ψpredawn and Ψmidday describes the relative sensitivity of the transpiration rate and plant hydraulic conductance to declining water availability. Two typical patterns, namely isohydricity and anisohydricity, have been described depending on the value of this parameter (Martinez-Vilalta et al., 2014). A slope equal to 1 indicates that plant transpiration is proportional to the hydraulic conductance, maintaining a similar daily pressure drop during drought and therefore a low or no regulation (anisohydricity). On the contrary, a lower slope than 1 is observed in tightly regulating species, i.e., isohydric. In other words, isohydric species should maintain Ψmidday at a higher level than anisohydric species in similar soil water status, and/or evaporative demand.
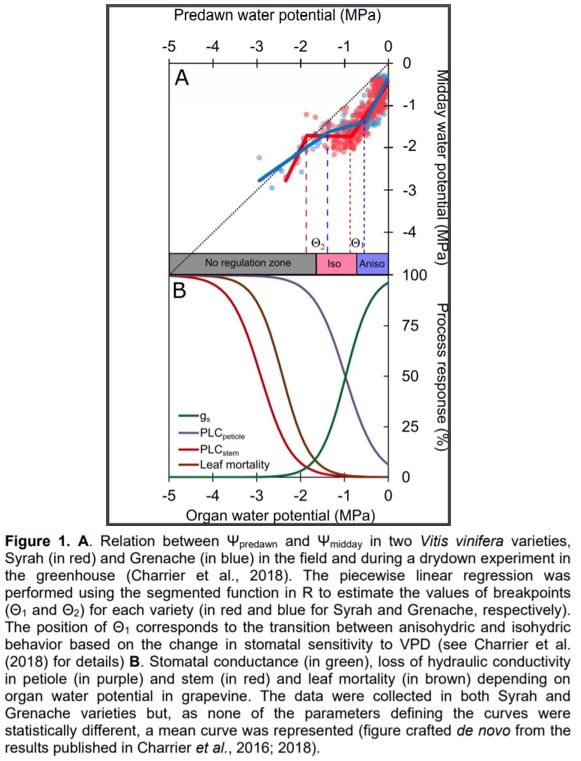 In this issue of Plant Physiology, Knipfer et al. (2020) revisited the correlation between Ψpredawn and Ψmidday, using a formula other than simple linear regression, such as the derivative of a smoothed function. Four distinct dry-down experiments, including slow (i.e., progressively decreasing irrigation) and rapid (i.e., irrigation stop) drying were carried out in three perennial crops (slow dry-down in almond [Prunus dulcis] and grapevine [Vitis vinifera], slow and rapid dry-down in walnut [Juglans regia]). They observed that slope is a dynamic value along the intensity of water stress, as previously shown by Charrier et al. (2018) in different grapevine varieties. As the level of stress increases, the behavior shifts from anisohydric (slope ≥ 1) to partially isohydric (slope < 1). Such dynamic behavior is supported by the change in stomatal sensitivity to Vapor Pressure Deficit (VPD) at similar Ψ (Charrier et al., 2018). Assuming a piecewise linear correlation between Ψpredawn and Ψmidday, Knipfer et al. observed two statistically significant breakpoints (Θ1 and Θ2) defining three distinct stages along the water stress gradient. During the first stage, a high transpiration rate can be achieved while the soil is above the saturation point: Ψleaf can thus decrease much faster than Ψsoil, resulting in a slope higher than 1. During the second stage, soil water becomes limiting, and Ψleaf reaches values that induce stomatal closure at midday, avoiding large pressure drops at midday, which results in a slope lower than 1. Finally, during the third stage, water losses can no longer be regulated and Ψleaf becomes equal to Ψsoil.
In this issue of Plant Physiology, Knipfer et al. (2020) revisited the correlation between Ψpredawn and Ψmidday, using a formula other than simple linear regression, such as the derivative of a smoothed function. Four distinct dry-down experiments, including slow (i.e., progressively decreasing irrigation) and rapid (i.e., irrigation stop) drying were carried out in three perennial crops (slow dry-down in almond [Prunus dulcis] and grapevine [Vitis vinifera], slow and rapid dry-down in walnut [Juglans regia]). They observed that slope is a dynamic value along the intensity of water stress, as previously shown by Charrier et al. (2018) in different grapevine varieties. As the level of stress increases, the behavior shifts from anisohydric (slope ≥ 1) to partially isohydric (slope < 1). Such dynamic behavior is supported by the change in stomatal sensitivity to Vapor Pressure Deficit (VPD) at similar Ψ (Charrier et al., 2018). Assuming a piecewise linear correlation between Ψpredawn and Ψmidday, Knipfer et al. observed two statistically significant breakpoints (Θ1 and Θ2) defining three distinct stages along the water stress gradient. During the first stage, a high transpiration rate can be achieved while the soil is above the saturation point: Ψleaf can thus decrease much faster than Ψsoil, resulting in a slope higher than 1. During the second stage, soil water becomes limiting, and Ψleaf reaches values that induce stomatal closure at midday, avoiding large pressure drops at midday, which results in a slope lower than 1. Finally, during the third stage, water losses can no longer be regulated and Ψleaf becomes equal to Ψsoil.
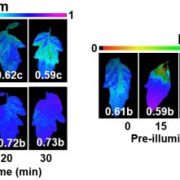
The correlation analysis was supplemented by experimental measurements to identify potential physiological causes for the identified thresholds (Θ1 and Θ2). As hypothesized, the first breakpoint (Θ1) was synchronized with Ψ inducing 90% stomatal closure (or Ψclose; Martin StPaul et al., 2017), in the different experiments (fast vs slow drying, and across species). In the first stage of dehydration (above Θ1), Ψleaf is affected by environmental conditions (i.e. light, temperature, and VPD) and does not necessarily reach Ψclose at midday. However, the identification of Ψsoil, which is likely to induce stomatal closure and the resulting loss of net assimilation (Θ1), is a relevant metric for physiologists, agronomists, and foresters. The second breakpoint (Θ2) was assumed to be the pressure at the turgor loss point (πtlp). This value should be considered with more caution, as the study reports only relative agreement between Θ2 and Ψtlp. The value for Ψtlp was measured in only one experiment using pressure–volume curves (walnut trees during slow dry down), while the other values were taken from the literature. Among species, Ψtlp is not necessarily observed at a lower value than Ψclose (Barltett et al., 2016), suggesting that the resulting shape of the correlation between Ψpredawn and Ψmidday would be different. An important factor explaining this discrepancy could be the variation in Ψtlp among different plant materials (e.g. genotype and phenological and physiological stage). In particular, diurnal variation in non-structural carbohydrates in leaves can significantly affect osmotic pressure and thus leaf turgor (Gersony et al., 2020; Charrier, 2020). Comparison between breakpoints and other physiological thresholds such as conductance and water content losses in different organ and tissues (e.g. stomata, leaf, petiole, stem, and root; Charrier et al., 2016; Lamacque et al., 2020; Scoffoni et al., 2017) provides a clearer picture of the sequence of events during drought stress. For example, in two grapevine varieties, Θ1 seems to be more related to the transition from anisohydric to isohydric behavior, whereas Θ2 would occur near the point of stomatal closure, complete petiole embolism, and loss of leaf conductance and the onset of stem embolism and leaf mortality (Figure 1). To ensure that the described pattern is generic, different components of plant water regulation should systematically be measured in various species and combined to the promising analytical approach presented by Knipfer et al..
References
Améglio, T., Archer, P., Cohen, M., Valancogne, C., Daudet, F. A., Dayau, S., & Cruiziat, P. (1999). Significance and limits in the use of predawn leaf water potential for tree irrigation. Plant and Soil, 207(2), 155-167.
Bartlett, M. K., Klein, T., Jansen, S., Choat, B., & Sack, L. (2016). The correlations and sequence of plant stomatal, hydraulic, and wilting responses to drought. Proceedings of the National Academy of Sciences, 113(46), 13098-13103.
Charrier, G. (2020). Gaining Sugars While Sweating: How Do Leaves Regulate Their Osmolarity?. Plant Physiology, 183(4) 1412-1413.
Charrier, G., Delzon, S., Domec, J. C., Zhang, L., Delmas, C. E., Merlin, I., … & Gambetta, G.A. (2018). Drought will not leave your glass empty: Low risk of hydraulic failure revealed by long-term drought observations in world’s top wine regions. Science advances, 4(1), eaao6969.
Charrier, G., Torres-Ruiz, J. M., Badel, E., Burlett, R., Choat, B., Cochard, H., … & Delzon, S. (2016). Evidence for hydraulic vulnerability segmentation and lack of xylem refilling under tension. Plant Physiology, 172(3), 1657-1668.
Dixon, H. H., & Joly, J. (1894). On the ascent of sap. Proceedings of the Royal Society of London, 57, 3-5.
Gersony, J. T., Hochberg, U., Rockwell, F. E., Park, M., Gauthier, P. P., & Holbrook, N. M. (2020). Leaf Carbon Export and Nonstructural Carbohydrates in Relation to Diurnal Water Dynamics in Mature Oak Trees. Plant Physiology, 183(4), 1612-1621.
Lamacque, L., Charrier, G., dos Santos Farnese, F., Lemaire, B., Ameglio, T., & Herbette, S. (2020). Drought-induced mortality: branch diameter variation reveals a point of no recovery in lavender species. Plant physiology, 183(4), 1638-1649.
Martin‐StPaul, N., Delzon, S., & Cochard, H. (2017). Plant resistance to drought depends on timely stomatal closure. Ecology letters, 20(11), 1437-1447.
Martínez‐Vilalta, J., Poyatos, R., Aguadé, D., Retana, J., & Mencuccini, M. (2014). A new look at water transport regulation in plants. New phytologist, 204(1), 105-115.
Scoffoni, C., Albuquerque, C., Brodersen, C. R., Townes, S. V., John, G. P., Bartlett, M. K., … & Sack, L. (2017). Outside-xylem vulnerability, not xylem embolism, controls leaf hydraulic decline during dehydration. Plant physiology, 173(2), 1197-1210.