Chloroplasts can Import Folded Proteins
Ganesan et al. investigate protein import into chloroplasts The Plant Cell (2018).
By Iniyan Ganesan and Steven Theg
Background: Chloroplasts are the green compartment in plant cells that carry out photosynthesis. Most plant proteins are made in the cytoplasm and many need to cross different cell membranes to reach their final cellular destination. Chloroplasts are surrounded by a double envelope membrane barrier that these proteins must cross. Whether the proteins are folded or unfolded during membrane transit through protein translocons is a fundamental question of cell biology. Proteins are known to be unfolded during import into mitochondria and the ER, but they can be folded during import into the nucleus and peroxisomes. We have long thought that proteins destined to the chloroplast are unfolded because a structural model protein, DHFR, was described to be unfolded during chloroplast import. However, solid evidence for this has not been forthcoming.
Question: We wanted to revisit the DHFR import experiment to see if folded proteins could be imported into chloroplasts. If so, the protein translocon pore must be relatively large. What is the actual size of the pore?
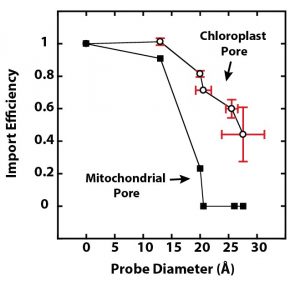
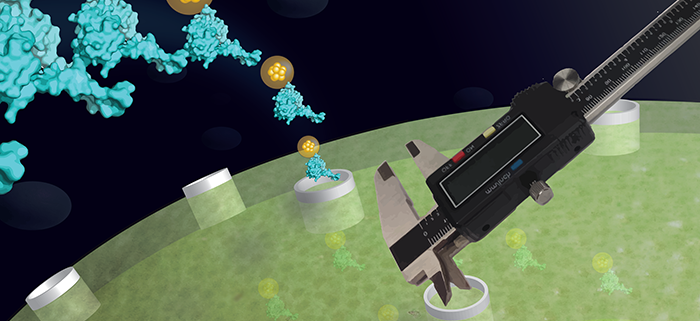
The chloroplast protein translocon pore is larger than the analogous pore in mitochondria.
Findings: We studied the import of proteins into chloroplasts by performing in vitro assays with isolated substrate proteins and isolated pea chloroplasts. Surprisingly, and contrary to previous conclusions, we found that DHFR substrate protein is imported into chloroplasts in a folded structure. We came to this conclusion because DHFR was imported in complex with a tightly binding fluorescent ligand, FMTX, and DHFR must remain folded to bind FMTX. Next, we measured the size of the protein translocon pore in the chloroplast envelope membranes by probing them with rigid particles chemically attached to substrate proteins. The pore was greater than 25.6 Å, but likely less than 30-35 Å. This size is large enough to accommodate folded DHFR. It is also significantly larger than the previously measured mitochondrial protein translocon pore that only transports unfolded proteins.
Next steps: The ability of chloroplasts to import folded proteins across a double membrane barrier is very unique. This finding will spur further interest in the protein import mechanism and translocon structure to understand how this feat is achieved. Another remaining question is why chloroplasts import folded proteins. Since the pore size is not large enough to accommodate all proteins in a folded conformation, which native chloroplast proteins are imported fully or partially folded?
Iniyan Ganesan, Lan-Xin Shi, Mathias Labs, Steven M. Theg. (2018). Evaluating the Functional Pore Size of Chloroplast TOC and TIC Protein Translocons: Import of Folded Proteins. Plant Cell 30: 2161-2173; DOI: https://doi.org/10.1105/tpc.18.00427