A Geminivirus Exploits the Host Machinery to Inhibit Methylation-mediated Defense Responses
Chen et al. uncover a strategy used by a DNA geminivirus to exploit the host machinery in order to inhibit methylation-mediated defense responses when establishing infection. The Plant Cell (2020) https://doi.org/10.1105/tpc.20.00249.
Background: Geminiviruses belong to one of the largest and most important families of plant viruses, with genomes comprising one or two single-stranded circular DNAs. Geminivirus genomes usually encode six or seven proteins that coordinate viral replication and virion assembly by regulating the timing of gene expression during infection in plants. DNA virus infection triggers both transcriptional and post-transcriptional gene silencing antiviral mechanisms. As a counter-defense strategy, geminiviruses have evolved multiple viral suppressors of RNA silencing (VSRs). To promote viral replication and counter-defense responses, the geminivirus early-class genes (Rep, C2, C3, C4) encoded by the complementary-sense strand are transcribed early during infection. By contrast, the virion-sense strand-encoded late-class genes (V1, V2, V3) are usually expressed after DNA replication and participate in virion assembly and movement.
Question: Much is known about how early-class viral gene products regulate late-class viral gene expression. However, less is known about the regulation of early genes and the host factors involved in this process.
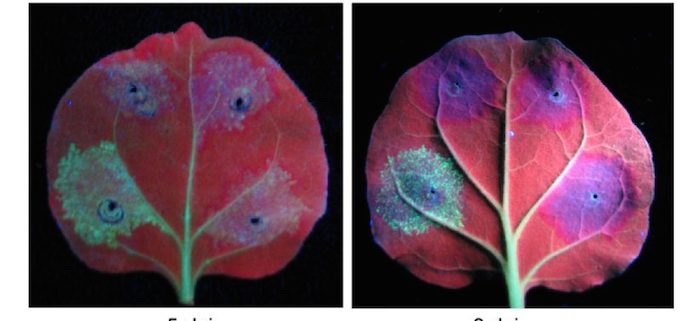
Findings: We found that infection by the geminivirus Beet severe curly top virus (BSCTV) induces the expression of VARIANT IN METHYLATION5 (VIM5), an endosperm imprinted gene that is conserved in diverse plant species. BSCTV-encoded Rep activates the expression of VIM5. VIM5 is a ubiquitin E3 ligase that directly targets the DNA methyltransferases MET1 and CMT3 for degradation by the ubiquitin-26S proteasome proteolytic pathway, leading to the early activation of C2 and C3 coupled with reduced symmetric methylation in the C2-C3 promoter and the onset of disease symptoms. These findings reveal that a virus-activated host E3 ligase participates in posttranslational regulation of DNA methyltransferases to facilitate the expression of the early-class C2 and C3 genes of a plant-infecting DNA virus.
Next steps: The discovery that a paternally imprinted gene activates viral gene expression suggests an innovative strategy used by viruses to screen plant genomes for host-susceptibility genes. We are investigating the mechanism by which VIM5 is induced in Arabidopsis and tomato by geminiviruses and the role of VIM5-mediated posttranslational regulation of DNA methyltransferases in embryo development.
Zhong-Qi Chen, Jian-Hua Zhao, Qian Chen, Zhong-Hui Zhang, Jie Li, Zhong-Xin Guo, Qi Xie, Shou-Wei Ding, and Hui-Shan Guo (2020). DNA Geminivirus Infection Induces an Imprinted E3 Ligase Gene to Epigenetically Activate Viral Gene Transcription. Plant Cell DOI: https://doi.org/10.1105/tpc.20.00249.