Tempting Fate: A Guanylate-Binding Protein Maintains Tomato Fruit Cell Differentiation
Tissues are composed of specialized cell types. As a cell matures, it acquires the characteristics associated with a specific identity, or fate. This process is called cell differentiation and is crucial for proper organ development. However, after a differentiation program is initiated, the mechanisms underlying its maintenance are often unclear. Here, Musseau et al. (2020) identified a new regulatory pathway that underlies cell fate maintenance in the fleshy fruit of tomato (Solanum lycopersicum).
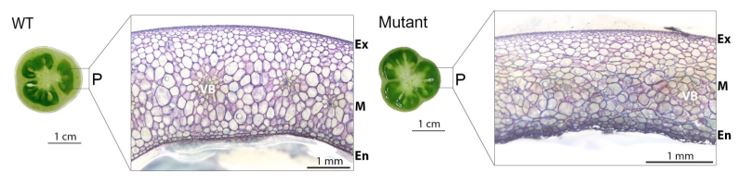
As a fruit develops, cells divide during early growth and enlarge during late growth prior to ripening (Renaudin et al., 2017). Tomato pericarp tissue acquires fleshy properties due to water and solute accumulation in enlarging mesocarp cells (see Figure). Mesocarp enlargement is also associated with endoreduplication, the replication of the nuclear genome in the absence of mitosis. To understand how these cell maturation programs are regulated, Musseau and colleagues characterized a tomato mutant isolated from a forward genetic screen (Musseau et al., 2017).
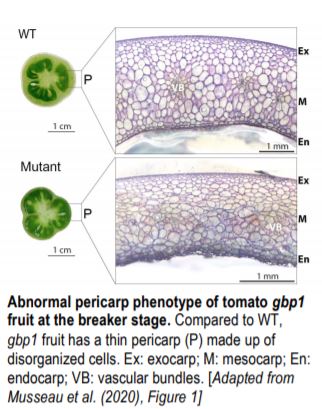 Compared to wild type (WT), the mutant exhibited reduced pericarp thickness (see Figure) but had no difference in the early production of pericarp layers, suggesting that late developmental events were affected. Mapping-by-sequencing indicated that a mutation in a gene encoding a GTPase Guanylate-binding protein, SlGBP1, caused the abnormal pericarp phenotype. Although GBPs have been described in humans and animals, their function in plants is poorly understood.
Compared to wild type (WT), the mutant exhibited reduced pericarp thickness (see Figure) but had no difference in the early production of pericarp layers, suggesting that late developmental events were affected. Mapping-by-sequencing indicated that a mutation in a gene encoding a GTPase Guanylate-binding protein, SlGBP1, caused the abnormal pericarp phenotype. Although GBPs have been described in humans and animals, their function in plants is poorly understood.
To determine how the mutant phenotype develops, the authors examined gbp1 fruit along a developmental time course. A higher proportion of small cells in the mesocarp suggested that the increase in cell expansion rate that normally takes place during the latter stages of development, beginning 20 days after a flower opens (days post anthesis; DPA), did not occur in gbp1 fruit.
Given that variations in tomato pericarp cell size are generally correlated with endoreduplication, the authors examined the nuclear ploidy levels of gbp1 pericarp cells. The ploidy index beginning at 20 DPA was much lower in the mutant compared to WT. Overall, the timing of endoreduplication was unaffected in gbp1 but a high proportion of cells stopped endocycling at a low ploidy state, suggesting that the process may be less efficient.
Another key factor that determines plant cell size and shape is cell wall rigidity. To examine cell wall differences between WT and gbp1, the authors examined the distribution and composition of pectins during fruit development. Together, the analyses suggested that cell wall loosening that takes place during WT fruit growth and ripening does not progress normally in gbp1 mesocarp. Comparison of metabolites between WT and gbp1 fruit pericarps also suggested that ripe gbp1 fruit is similar to developing WT fruit prior to ripening.
In addition to changes in wall composition, new cell walls formed after 20 DPA in gbp1, which resulted from divisions of large mesocarp cells. Changes in temporal expression patterns of known endocycle and mitotic cycle regulators further supported the notion that cell division activity resumed during late gbp1 fruit growth at the expense of endoreduplication.
It is generally accepted that entrance into the endocycle marks the start of cell differentiation. However, polyploid mesocarp cells of the gbp1 mutant deviate from this developmental program and exhibit cell divisions during late stages of fruit growth. Thus, SlGBP1 may be an inhibitor of cell division, a function comparable to that of human hGBP-1 protein. These results reveal a previously unknown role for GBPs in the control of cell cycle genes in plants. Taken together, Musseau and colleagues identified a new fate maintenance pathway that is required for proper cell differentiation and organ development.
Rachel Shahan
Department of Biology
Duke University
Durham, North Carolina
ORCID: 0000‐0001‐7702‐9432
REFERENCES
Musseau C, Just D, Jorly J, Gévaudant F, Moing A, Chevalier C, Lemaire-Chamley M, Rothan C, Fernandez L (2017). Identification of two new mechanisms that regulate fruit growth by cell expansion in tomato. Front. Plant Sci. 8, 1-15.
Renaudin J-P, Deluche C, Cheniclet C, Chevalier C, Frangne N (2017). Cell layer-specific patterns of cell division and cell expansion during fruit set and fruit growth in tomato pericarp. J. Exp. Bot. 68, 1613-1623.