A novel regulator of stomatal immunity in tomato
By Marcelo Lattarulo Campos
Integrative Plant Research Laboratory, Departamento de Botânica e Ecologia, Instituto de Biociências, Universidade Federal de Mato Grosso, Cuiabá/MT, Brazil.
ORCID ID: 0000-0001-6235-5120
The ability of a pathogen to successfully colonize a plant is determined in large part by its capacity to rapidly enter the host tissue. While most fungal pathogens secrete cuticle- and cell wall-degrading enzymes or use mechanical force to breach the epidermis (Mendgen et al., 1996), phytopathogenic bacteria are devoid of mechanisms that allow them to penetrate plant tissues. For this reason, bacterial aggressors rely on lenticels, root cracks, wounds and other natural surface openings to gain access to their hosts. Stomata also provide a natural entry point for bacteria. Leaf tissues can become vulnerable to bacterial invasion during periods of intense photosynthetic activity and transpiration, as these physiological processes depend on widely open stomata. To hinder bacterial invasion through these pores, plants have evolved sophisticated mechanisms that perceive bacterial attack and close their stomata, a defense strategy usually referred to as stomatal immunity (Sawiski et al., 2013). Little is known about the molecular elements involved in stomatal immunity in economically relevant plant-pathogen systems, as most of our knowledge on this topic comes from studies using the plant model Arabidopsis thaliana and phytopathogenic bacteria Pseudomonas syringae.
 In this issue of Plant Physiology, Guzman et al. (2020) shed light on this topic by uncovering the role of a novel regulator of stomatal immunity, the Tomato Atypical Receptor Kinase1 (TARK1). TARK1 is a leucine-rich repeat receptor-like kinase (LRR-RLK) that was previously identified by the same research group as a target for virulence factors produced by Xanthomonas euvesicatoria (Xe), the causal agent of bacterial spot disease in economically important plants such as tomato (Solanum lycopersicum) and peppers (Capsicum annuum) (Kim et al., 2009). Although their earlier work suggested a role for TARK1 in tomato immunity against Xe infection, its function remained elusive. Guzman et al. (2020) used an immunoprecipitation-proteomics approach to identify TARK1-associated protein complexes. They generated tomato plants overexpressing a GFP-fused TARK1 (TARK1 OE) and inoculated TARK1 OE plants with high doses of Xe DhrcV, a mutant variant of Xe that elicits a stronger defense response. TARK1 protein levels increased upon infection with Xe DhrcV even in the TARK1 OE line. Twenty-four hours after Xe DhrcV inoculation, the authors isolated TARK1-GFP and any associated proteins from infected tomato leaves. They used liquid chromatography-mass spectrometry to identify 17 candidate proteins that associate with TARK1 upon Xe infection. Among these candidates, three proteins were notable for their putative involvement in stomatal immunity: the plasma membrane H+-ATPase HA4, the lipoxygenase LOX8, and an LRR-RLK referred to as RLK15. Coimmunoprecipitation assays confirmed that TARK1 interacts with HA4 and RLK15, but not with LOX8. Together, these results suggested that TARK1 may be involved in stomatal immunity in tomato.
In this issue of Plant Physiology, Guzman et al. (2020) shed light on this topic by uncovering the role of a novel regulator of stomatal immunity, the Tomato Atypical Receptor Kinase1 (TARK1). TARK1 is a leucine-rich repeat receptor-like kinase (LRR-RLK) that was previously identified by the same research group as a target for virulence factors produced by Xanthomonas euvesicatoria (Xe), the causal agent of bacterial spot disease in economically important plants such as tomato (Solanum lycopersicum) and peppers (Capsicum annuum) (Kim et al., 2009). Although their earlier work suggested a role for TARK1 in tomato immunity against Xe infection, its function remained elusive. Guzman et al. (2020) used an immunoprecipitation-proteomics approach to identify TARK1-associated protein complexes. They generated tomato plants overexpressing a GFP-fused TARK1 (TARK1 OE) and inoculated TARK1 OE plants with high doses of Xe DhrcV, a mutant variant of Xe that elicits a stronger defense response. TARK1 protein levels increased upon infection with Xe DhrcV even in the TARK1 OE line. Twenty-four hours after Xe DhrcV inoculation, the authors isolated TARK1-GFP and any associated proteins from infected tomato leaves. They used liquid chromatography-mass spectrometry to identify 17 candidate proteins that associate with TARK1 upon Xe infection. Among these candidates, three proteins were notable for their putative involvement in stomatal immunity: the plasma membrane H+-ATPase HA4, the lipoxygenase LOX8, and an LRR-RLK referred to as RLK15. Coimmunoprecipitation assays confirmed that TARK1 interacts with HA4 and RLK15, but not with LOX8. Together, these results suggested that TARK1 may be involved in stomatal immunity in tomato.
To test the hypothesis that TARK1 is involved in stomatal movement, Guzman et al. (2020) employed CRISPR Cas9-mediated genome editing to create a loss-of-function TARK1 knockout line, TARK1 CR. They monitored stomatal movement in WT, TARK1 OE, and TARK1 CR lines upon treatment with different stimuli known to induce stomatal opening or closure such as dark-to-light transition and treatments with abscisic acid (ABA), salicylic acid (SA), the bacterial flagellin peptide Flg22, and the fungal polysaccharide chitin. No difference in stomatal behavior was observed among the genotypes when treated with dark-to-light transition, ABA, or chitin, indicating that TARK1 is not required for light-induced stomatal opening nor for ABA- and chitin-induced stomatal closure. On the other hand, TARK1 CR lines appear to be more sensitive to SA and Flg22 treatment, as stomata were significantly more closed in response to these two treatments compared to wild-type (WT) and TARK1 OE plants. As SA and Flg22 are fundamental components of signaling networks involved in plant defense responses against bacterial pathogens (Yi & Kwon et al., 2014), these results point to the involvement of TARK1 in bacteria-induced stomatal closure.
One of the most fascinating aspects of stomatal immunity is the observation that many phytopathogenic bacteria can secrete virulence factors such as coronatine (COR) to reopen stomata (Melotto et al., 2008). Given the evidence that TARK1 may be involved in bacterial-induced stomatal closure, Guzman et al. (2020) tested whether stomatal reopening was affected in TARK1 OE and TARK1 CR lines. They monitored stomatal behavior in plants treated with suspensions of P. syringae and with a mutant strain of the pathogen, DC3118, which cannot produce COR, to stimulate stomatal reopening. In WT tomato plants, stomata close 1 h after P. syringae treatment and reopen 4 h after P. syringae treatment in a COR-dependent manner. In TARK1 CR, the stomatal response to bacteria-delivered COR was impacted, as although the stomata of these lines closed 1 h after P. syringae treatment, they showed no reopening 4 h post P. syringae treatment (with either WT or DC3118 strains). TARK1 OE lines showed no stomatal closure at either timepoint when challenged with each strain of P. syringae. Together, these results suggest that TARK1 plays a critical role in stomatal closure and reopening in response to phytopathogenic bacteria treatment.
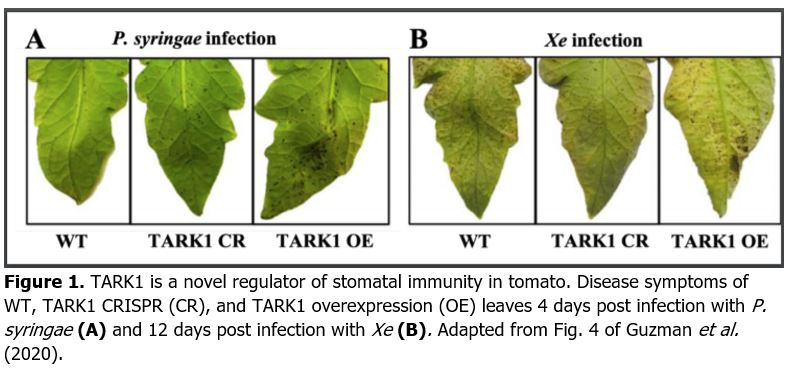
To evaluate the direct role of TARK1 in defense responses against phytopathogenic bacteria, Guzman et al. (2020) then assessed bacterial growth and disease symptoms in TARK CR and TARK OE lines spray-inoculated with P. syringae and Xe. For both pathogens, TARK1 OE leaflets showed more severe disease symptoms with a significantly higher number of bacteria (Fig. 1A and B) compared to WT. On the other hand, Xe bacterial spot symptoms were milder for TARK1 CR than WT (Figure 1B), although bacterial growth was similar on both genotypes. The increased susceptibility of TARK1 OE lines is likely explained by the failure to close stomata upon challenge with phytopathogenic bacteria, allowing higher bacterial titers to penetrate the apoplast and cause disease. Indeed, confirmation that these differences in bacterial resistance are dependent on stomatal immunity comes from the observation that bacterial growth was the same for all lines when the P. syringae was hand-injected inside the plant apoplast, thus bypassing the stomatal barrier. This observation is clear evidence that TARK1 plays a critical role in stomatal defense against bacterial pathogens in tomato.
Humans have been dealing with phytopathogenic bacteria since we began domesticating plants and these microbial aggressors remain a major obstacle to reliable and secure food production (Savary et al., 2019). Evidence suggests that relentless selection for important agronomical traits was accompanied by a massive reduction in the capacity of domesticated plants to defend themselves against pests and pathogens (Chen et al., 2015; Moreira et al., 2018). Thus, it is imperative that we decipher the molecular basis of mechanisms in economically important plants (such as tomato) to fend off bacterial attack. Work by Guzman et al. (2020) has uncovered a novel regulator of stomatal immunity in tomato, TARK1. Although future work is needed to determine the exact mechanism of TARK1 function and how it participates in stomatal defense, this study provides important future avenues for the development of pathogen-resistant crops.
LITERATURE CITED
Chen YH, Gold R, Benrey B (2015). Crop domestication and its impact on naturally selected trophic interactions. Annu Rev Entomol 60:3.21-3.24.
Guzman AR, Kim JG, Taylor KW, Lanver D, Mudgett MB (2020). Tomato Atypical Receptor Kinase1 is involved in the regulation of pre-invasion defense. Plant Physiol https://doi.org/10.1104/pp.19.01400
Kim JG, Li X, Roden JA, Taylor KW, Aakre CD, Su B, Lalonde S, Kirik A, Chen Y, Baranage G et al. (2009). Xanthomonas T3S effector XopN suppresses PAMP-triggered immunity and interacts with a Tomato Atypical Receptor-Like Kinase and TFT1. Plant Cell 21:1305-1323.
Melotto M, Underwood W, He SY (2008). Roles of stomata in plant innate immunity and foliar bacterial diseases. Annu Rev Phytopathol 46:101-122.
Mendgen K, Hahn M, Deising H (1996). Morphogenesis and mechanisms of penetration by plant pathogenic fungi. Annu Rev Phytopathol 34:367-386.
Moreira X, Abdala-Roberts L, Gols R, Francisco M (2018). Plant domestication decreases both constitutive and induced chemical defences by direct selection against defensive traits. Sci Rep 8:12678.
Savary S, Willocquet L, Pethybridge SJ, Esker P, McRoberts N, Nelson A (2019). The global burden of pathogens and pests on major food crops. Nat Ecol Evol 3:430-439.
Sawinski K, Mersmann S, Robatzek S, Bohmer M (2013). Guarding the green: Pathways to stomatal immunity. Mol Plant Microb Interact 26:626-632.
Yi SY Kwon SY (2014). How does SA signaling link the Flg22 responses? Plant Signal Behav 9:e972806.