Structural basis of unidirectional energy transfer in Porphyridium purpureum phycobilisome (Nature)
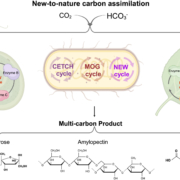
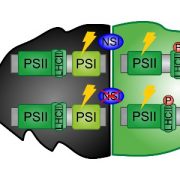
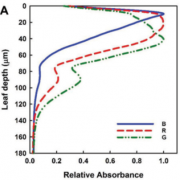
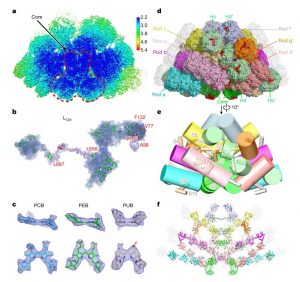 Cyanobacteria and red algae employ phycobilisomes (PBSs) as light-harvesting systems to adapt to fluctuating environments. PBSs are composed of phycobiliproteins, linker proteins and chromophores. Ma et al. used cryo-EM to determine the 2.82 Å structure of the very large (14.7-megadalton, 706 protein subunit) PBS from the unicellular reg alga Porphyridium purpureum, which they compared to that of a previously characterized PBS from the multicellular red alga Griffithsia pacifica. The newly characterized PBS complex contained 528 phycoerythrin, 72 phycocyanin, 46 allophycocyanin, 60 linker proteins and 1598 chromophores. The structure provides insights into how the energy states of the chromophores can be affected through interacting with the aromatic amino acids of the linker proteins, therefore ensuring the efficient unidirectional transfer of energy within phycobilisome. The study shows how light is harvested and transferred in the PBS of cyanobacteria and red algae, and will benefit further study of artificial photosynthesis. (Summary by Nanxun Qin) Nature 10.1038/s41586-020-2020-7
Cyanobacteria and red algae employ phycobilisomes (PBSs) as light-harvesting systems to adapt to fluctuating environments. PBSs are composed of phycobiliproteins, linker proteins and chromophores. Ma et al. used cryo-EM to determine the 2.82 Å structure of the very large (14.7-megadalton, 706 protein subunit) PBS from the unicellular reg alga Porphyridium purpureum, which they compared to that of a previously characterized PBS from the multicellular red alga Griffithsia pacifica. The newly characterized PBS complex contained 528 phycoerythrin, 72 phycocyanin, 46 allophycocyanin, 60 linker proteins and 1598 chromophores. The structure provides insights into how the energy states of the chromophores can be affected through interacting with the aromatic amino acids of the linker proteins, therefore ensuring the efficient unidirectional transfer of energy within phycobilisome. The study shows how light is harvested and transferred in the PBS of cyanobacteria and red algae, and will benefit further study of artificial photosynthesis. (Summary by Nanxun Qin) Nature 10.1038/s41586-020-2020-7
[altmetric doi=”10.1038/s41586-020-2020-7″ details=”right” float=”right”]