Protein Editing for Multi-Tasking
Zauner et al. investigate the mechanism of protein re-purposing. The Plant Cell (2018). https://doi.org/10.1105/tpc.17.00963.
By Florian B. Zauner, Elfriede Dall and Hans Brandstetter
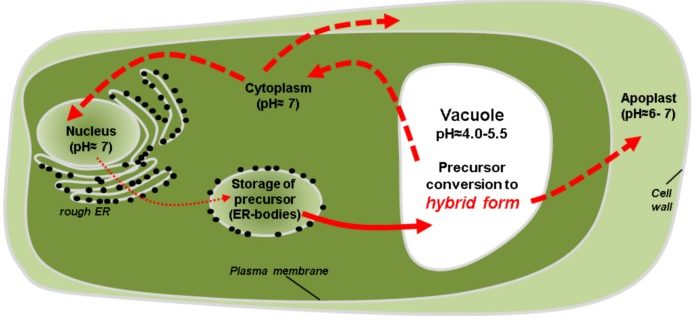
Background: Plants cannot run away from herbivores, drought, or heat. To withstand adverse conditions, plants have developed a diverse arsenal of proteins, their proteomes (total complement of proteins). Certain enzymes function to edit or modify other proteins in order to diversify their functions upon demand. Legumains are one such enzyme family which combine protease and peptide ligase activities, enabling the recombination of target proteins upon demand. How legumains themselves adapt to diverse environments, such as different pH conditions, was unclear, because their protease and ligase activities appeared to be incompatible with their stability.
Question: We wanted to know how legumains regulate their complementary editing activities and how they adapt to different environmental factors such as acidic and neutral pH or variations in protein concentration.
Findings: We found that legumains can adopt three different forms, an inactive precursor form, a fully active enzyme form, and a newly discovered hybrid form which can either be inactive or active, depending on environmental factors. The hybrid form confers flexibility to legumain with respect to enhanced stability and modulation of activity.
Next steps: Their unique properties allow legumains, for example, to convert a storage protein into an enzyme inhibitor, serving as a defense weapon against hostile proteases. Legumains have been reported to exhibit diverse and apparently incompatible functions, including transcriptional control in the production of proteins and inducing programmed cell death as an immune defense. We aim to test how the hybrid form of legumain can reconcile these properties that appeared conflicting in the simple model of inactive precursor and fully active enzyme forms of legumain.
Florian B. Zauner, Elfriede Dall, Christof Regl, Luigi Grassi, Christian G. Huber, Chiara Cabrele and Hans Brandstetter (2018). Crystal structure of plant legumain reveals a unique two-chain state with pH-dependent activity regulation. https://doi.org/10.1105/tpc.17.00963.