Clathrin-Mediated Endocytosis: Plant Homologs of the Clathrin Uncoating Factor Auxilin
Adamowski et al. use CRISPR to investigate the function of plant auxilin proteins https://doi.org/10.1105/tpc.17.00785.
By Maciek Adamowski
Background: Endocytosis is one of the basic pathways of cellular trafficking. By endocytosis, proteins located in the plasma membranes, for instance receptors, can be taken up into the cell. In plants, the best known mechanism of endocytosis is based on the protein clathrin. The process of clathrin-mediated endocytosis involves generation of small vesicles at the plasma membrane. These vesicles are surrounded by a coat of clathrin and other associated proteins. The mechanism of clathrin-mediated endocytosis has been studied extensively in animals and yeast, but much less in plants.
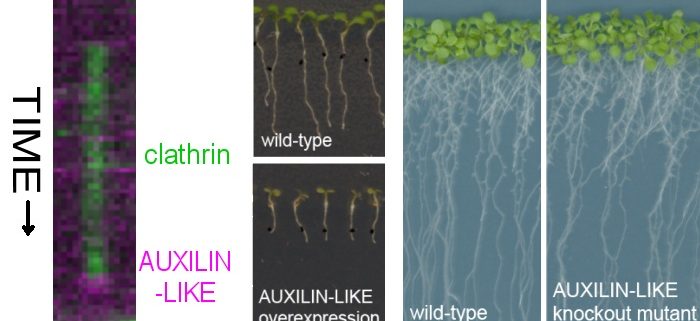
Question: According to researchers working on endocytosis in yeast and animals, after the complete vesicle is released from the plasma membrane during final steps of clathrin-mediated endocytosis, the coat of clathrin and other associated proteins, which surrounds the lipid vesicle, is removed. Uncoating is performed by a protein called auxilin together with a protein chaperone called Hsc70. So far, the homologs for uncoating machinery in plants have not been well-characterized. In this work, with protein-protein interaction methods, we set out to find new proteins involved in clathrin-mediated endocytosis in the model plant Arabidopsis thaliana. We found two potential homologs of the uncoating protein auxilin, which we named AUXILIN-LIKE1 and AUXILIN-LIKE2, and began their functional characterization.
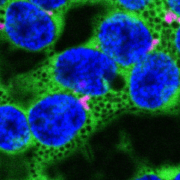
Findings: First, we overexpressed AUXILIN-LIKE1/2 in A.thaliana. The overexpressing plants were strongly affected in their growth, clathrin did not bind to the plasma membrane, and endocytosis was blocked. With CRISPR technology, we also isolated auxilin-like1/2 loss-of-function mutants. In contrast to overexpressing plants, and to our surprise, knock-out mutants grew and developed normally and had functional endocytosis. By fluorescent live microscopy, we studied the dynamics of AUXILIN-LIKE proteins in normal cells. We saw that AUXILIN-LIKEs bind to the clathrin-coated vesicle just before it leaves the plasma membrane, in the same way as was previously described in animal cells. But AUXILIN-LIKEs were only recruited to a small fraction of all forming vesicles, while in animals, auxilins are recruited to all clathrin-coated vesicles. Perhaps, in plants, most clathrin-coated vesicles are not uncoated by auxilin and Hsc70. This idea would also explain why auxilin-like1/2 double mutant were still able to perform endocytosis and develop normally.
Next Steps: We would like to proceed further towards understanding the exact molecular function of AUXILIN-LIKE1/2 in plants. It is possible that its function is required for endocytosis of a particular cargo protein, while not for all endocytic events. To realize this, we will study the auxilin-like1/2 mutant in more detail, and perform further protein-protein interaction studies. This will be a step towards a better understanding of the endocytic process in plants, which, as we can already see, differs from endocytosis in yeast or animals.
Maciek Adamowski, Madhumitha Narasimhan, Urszula Kania, Matouš Glanc, Geert De Jaeger, Jiří Friml (2018) Plant Cell. Published March 2018. DOI: https://doi.org/10.1105/tpc.17.00785
keywords: clathrin, endocytosis, trafficking, cell biology