An Emerging Paradigm? RxLR Cleavage Before Effector Secretion
Eukaryotic pathogens are responsible for devastating plant diseases that threaten food supplies globally – think potato blight caused by the oomycete Phytophora infestans, rice blast caused by the fungus Magnaporthe oryzae, and wheat stem rust caused by the fungus Puccinia graminis f. sp. tritici. These pathogens secrete effector proteins that condition the host cells for successful infection, some by acting in the apoplast and others after entering into the host cells. Many oomycete effectors have an RxLR sequence motif in their N-terminal region that seems to function in host cell targeting, although the mechanisms are a matter of debate (reviewed in Wawra et al., 2012 and Petre and Kamoun, 2014). It is notoriously difficult to study the secretion and targeting of effectors, as these processes occur only at the interface of the pathogen with the host and only during infection. In fact, there are mounting indications that some alternative approaches often used to assess pathogen effector secretion and entry in the host plant could be flawed (see, for example, Petre et al., http://dx.doi.org/10.1101/038232). In a new Breakthrough Report, Wawra & Trusch et al. (2017) provide evidence that the RxLR motif is important for effector secretion from the pathogen, rather than for direct interaction with the host cells.
Plasmodium parasites, which cause malaria, are distantly related to oomycete plant pathogens and similarly have RxLR-like N-terminal sequences that are responsible for targeting to host cells. For many Plasmodium effectors, this so-called PEXEL motif is cleaved in the ER, after which the newly exposed N-terminus is acetylated and the effector is secreted. Another motif, called the TEXEL motif, is important for effector processing in Toxoplasma gondii. The similarity of the RxLR motif to the PEXEL and TEXEL motifs prompted Wawra and coworkers to explore whether RxLR cleavage is involved in effector secretion from P. infestans.
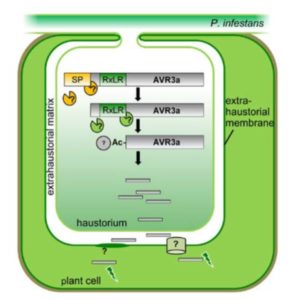 Wawra & Trusch et al. isolated the native (untagged) form of the AVR3a effector secreted into the culture medium by P. infestans. LC-MS/MS analysis did not reveal a peptide representing any sequence more N-terminal than the RxLR motif, but did find a peptide that started immediately downstream of the motif. In addition, the MS data showed likely acetylation. To further characterize the secreted form of the effector, the authors performed reverse-phase chromatography followed by MALDI-TOF analysis. After deglycosylation, the mass of the main product indicated that the AVR3a protein from the medium lacked the first 47 amino acids (i.e., the region up to and including the RxLR motif) with an additional mass for a possible acetylation.
Wawra & Trusch et al. isolated the native (untagged) form of the AVR3a effector secreted into the culture medium by P. infestans. LC-MS/MS analysis did not reveal a peptide representing any sequence more N-terminal than the RxLR motif, but did find a peptide that started immediately downstream of the motif. In addition, the MS data showed likely acetylation. To further characterize the secreted form of the effector, the authors performed reverse-phase chromatography followed by MALDI-TOF analysis. After deglycosylation, the mass of the main product indicated that the AVR3a protein from the medium lacked the first 47 amino acids (i.e., the region up to and including the RxLR motif) with an additional mass for a possible acetylation.
Wawra and coworkers followed up on the possibility of N-terminal acetylation using Edman degradation, which can cleave an N-terminal peptide bond when it is accessible. They observed no cleavage of the AVR3a peptide from the medium, indicating that the N-terminus was not accessible, consistent with it being acetylated. The likely N-acetylation of AVR3a is particularly intriguing as the acetyltransferases that carry out such N-acetylation are found only inside the cell.
Overall, these results are consistent with cleavage of the RxLR motif of AVR3a, followed by acetylation of the new N-terminal amino acid before secretion from P. infestans. It is not clear what protease might be responsible for the cleavage, because none of the 11 P. infestans aspartic proteases homologous to the protease that cleaves the PEXEL motif in Plasmodium could cleave AVR3a in assays using recombinant bacterially expressed proteins. Nevertheless, the potential similarity of this process to the processing and secretion of effectors from other species containing the PEXEL and TEXEL motifs points to its biological relevance and a possible conserved mechanism for effector secretion.
IN BRIEF by Nancy R. Hofmann [email protected]
REFERENCES
Petre, B., and Kamoun, S. (2014). How do filamentous pathogens deliver effector proteins into plant cells? PLoS Biol 12, e1001801.
Wawra, S., Belmonte, R., Lobach, L., Saraiva, M., Willems, A., and van West, P. (2012a). Secretion, delivery and function of oomycete effector proteins. Curr Opin Microbiol 15, 685-691.
Wawra, S., Trusch F., Matena, A., Apostolakis, K., Linne, U., Zhukov, I., Stanek, J., Koźmiński, W., Davidson, I., Secombes, C.J, Bayer, P. and van West, P. (2017). The RxLR Motif of the Host Targeting Effector AVR3a of Phytophthora infestans Is Cleaved Before Secretion. Plant Cell. doi: 10.1105/tpc.16.00552.





Leave a Reply
Want to join the discussion?Feel free to contribute!