Peripheral? Not Really! The Extracellular Arabinogalactan Proteins Function in Calcium Signaling
Arabinogalactan proteins (AGPs) are a family of extracellular proteoglycans that existed in land plants and algae (Johnson et al., 2017). AGPs are part of the hydroxyproline-rich glycoprotein (HRGP) superfamily that also includes extensins and proline-rich proteins. Based on the protein sequence, a glycosylphosphatidylinositol (GPI) anchor is predicted to be present at the C-terminus of many AGPs and is expected to attach the AGPs to the extracellular side of the plasma membrane, thereby forming a narrow AGP-rich region between the cell membrane and the cell wall proper. AGPs are highly decorated with arabinogalactan polysaccharides (AGs) O-linked to the hydroxyproline residues of the protein core. Over 100 proteins are predicted to carry AGs (Borner et al., 2003). Due to the chemical similarity of their carbohydrate moieties and functional redundancy amongst members of this family, studies of AGPs have been challenging and, consequently, little is known about the general molecular function of AGPs (Tan et al., 2012).
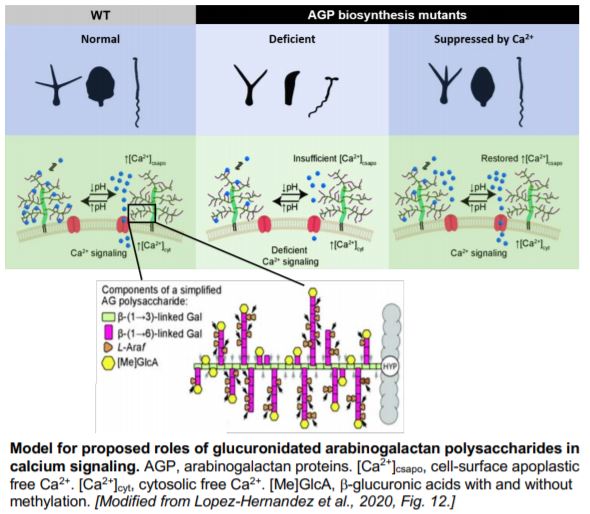 The arabinogalactan polysaccharides of AGPs are composed of a distinctive b-(1®3)-galactan backbone that is further substituted by b-(1®6)-galactan side chains. Glucuronidation is often found at both the backbone and the side chains of AGs. The glucuronic acids of AGPs have been shown in vitro to bind calcium in a pH-dependent manner, leading to the “AGP-Ca2+ capacitor” hypothesis (Lamport and Várnai, 2013). This hypothesis proposes that glucuronidated AGPs act as a Ca2+ reservoir at the cell-surface apoplast and provide the primary source for cellular Ca2+ oscillations, thus coordinating Ca2+ signals with plant growth, development and other physiological events. In a recent study, Lopez-Hernandez et al. (2020) tested aspects of this hypothesis and addressed the role of AGP glucuronidation in vivo using Arabidopsis thaliana mutants.
The arabinogalactan polysaccharides of AGPs are composed of a distinctive b-(1®3)-galactan backbone that is further substituted by b-(1®6)-galactan side chains. Glucuronidation is often found at both the backbone and the side chains of AGs. The glucuronic acids of AGPs have been shown in vitro to bind calcium in a pH-dependent manner, leading to the “AGP-Ca2+ capacitor” hypothesis (Lamport and Várnai, 2013). This hypothesis proposes that glucuronidated AGPs act as a Ca2+ reservoir at the cell-surface apoplast and provide the primary source for cellular Ca2+ oscillations, thus coordinating Ca2+ signals with plant growth, development and other physiological events. In a recent study, Lopez-Hernandez et al. (2020) tested aspects of this hypothesis and addressed the role of AGP glucuronidation in vivo using Arabidopsis thaliana mutants.
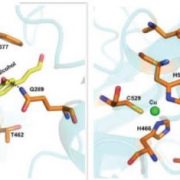
With bioinformatics analysis, the authors identified and chose four AG b-glucuronyltransferases (GlcAT14A, B, D and E) for further mutant studies. AG glucuronidation was significantly reduced in the glcat14a/b double mutant, and the glcat14a/b/d and glcat14a/b/e triple mutants. The abundance of extracted glucuronidated oligosaccharides of different lengths varied among these mutants, suggesting varied substrate preferences of different GlcATs. The AGs from glcat14a/b/d mutants bound about 80% less Ca2+ in vitro compared to those from wild-type plants.
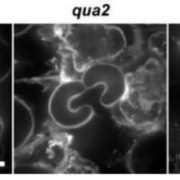
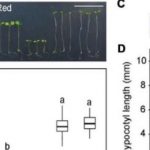
The reduction in AG glucuronidation caused pleiotropic growth defects such as shorter inflorescence stems and reduced trichome branching. The glcat14a/b/e mutants displayed a de-etiolated phenotype in dark-grown hypocotyls. However, increasing the concentration of calcium in the growth medium suppressed these developmental phenotypes and this suppression appeared to be specific to calcium and not magnesium. Finally, calcium waves induced by H2O2 were perturbed in the roots of AG glucuronidation mutants, indicating altered intracellular calcium signals. Together these results provide a solid step towards validating the AGP-Ca2+ capacitor hypothesis, casting light on the molecular roles of AGPs in plant growth and development.
Tian Zhang
Assistant Features Editor
ORCID: 0000-0002-0453-1621
Borner, G.H.H., Lilley, K.S., Stevens, T.J., and Dupree, P. (2003). Identification of Glycosylphosphatidylinositol-Anchored Proteins in Arabidopsis. A Proteomic and Genomic Analysis. Plant Physiol. 132: 568–577.
Johnson, K.L. et al. (2017). Insights into the evolution of hydroxyproline-rich glycoproteins from 1000 plant transcriptomes. Plant Physiol. 174: 904–921.
Lamport, D.T.A. and Várnai, P. (2013). Periplasmic arabinogalactan glycoproteins act as a calcium capacitor that regulates plant growth and development. New Phytol. 197: 58–64.
Tan, L., Showalter, A., Egelund, J., Hernandez-Sanchez, A., Doblin, M., and Bacic, A. (2012). Arabinogalactan-proteins and the research challenges for these enigmatic plant cell surface proteoglycans. Front. Plant Sci. 3: 140.