Let’s stick together – a pectin biosynthetic mutant reveals the interconnectedness of plant cell walls
The plant cell wall is vital for plant survival—it mediates defence against pathogens, provides structural stability and controls growth to produce final plant form. Plant cell walls are composite structures formed mainly from cellulose, hemicelluloses, and pectins, and the interactions of these components produce a complex structure that fulfils all these needs at once. But how these interactions affect cell wall structure is not well understood, nor are the genes that act to synthesize and modify cell wall components, in particular pectins, which control wall thickness, cell adhesion and tissue integrity.
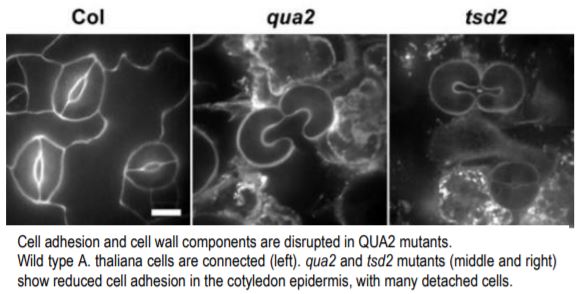 In this issue of The Plant Cell, first author Juan Du and colleagues explore how pectins affect cell wall properties by investigating the role of QUASIMODO2 (QUA2), a Homogalacturonan (HG) methyltransferase in Arabidopsis thaliana that has long been hypothesized to be involved in pectin biosynthesis (Du et al., 2020). They characterize QUA2 function and show mutations in this gene affect not just pectin, but also cellulose and even the cytoskeleton, highlighting the complex and interconnected nature of the plant cell wall.
In this issue of The Plant Cell, first author Juan Du and colleagues explore how pectins affect cell wall properties by investigating the role of QUASIMODO2 (QUA2), a Homogalacturonan (HG) methyltransferase in Arabidopsis thaliana that has long been hypothesized to be involved in pectin biosynthesis (Du et al., 2020). They characterize QUA2 function and show mutations in this gene affect not just pectin, but also cellulose and even the cytoskeleton, highlighting the complex and interconnected nature of the plant cell wall.
The authors began by expressing a tagged version of QUA2 in E. coli and purifying the protein. They showed that QUA2 can methylate HG in vitro, suggesting that QUA2 is indeed a functional HG methyltransferase. To understand how this molecular function impacts the whole plant, they analyzed two QUA2 mutants, qua2 and tumorous shoot development 2 (tsd2), both of which produce truncated proteins. They showed that qua2 and tsd2 both produce smaller plants and contain epidermal cells in the hypocotyl and cotyledon that are often detached from one another (see figure). This confirms previous work that intercellular adhesion is reduced in QUA2 mutants (Krupkova et al., 2007; Mouille et al., 2007).
To understand how QUA2 affects cell wall composition in planta, the authors analyzed uronic acid content and polysaccharide composition of the mutants. They showed that uronic acid content is lower, but that although there was less HG in the wall, the degree of HG methylesterification was unchanged. This suggests that other methyltransferases also control HG methylesterification, and that QUA2 mutants might synthesize an unstable or incomplete form of HG that is rapidly degraded, causing lower HG levels.
To test whether disruption of pectin biosynthesis affects other cell wall components, the authors measured cellulose content of qua2 and tsd2 mutants and showed that it is significantly reduced. They then visualized cellulose synthase complexes (CSCs) by GFP tagging. CSCs move along microtubule tracks to produce cellulose microfibrils, and the authors found that GFP-tagged cellulose synthase particles moved significantly slower in the mutants than in wild type seedlings. They also used Field Emission Scanning Electron Microscopy and Atomic Force Microscopy to characterize cell wall structure and showed that both mutants contain disordered fibril arrangements in their cell walls. These data suggest that disrupting pectin biosynthesis has knock-on effects for other cell wall components and disrupts cellulose synthesis and organisation.
They then investigated whether the altered wall structure in QUA2 mutants can disrupt cortical microtubules, which are necessary for the patterned deposition of cellulose in the wall. They visualized GFP-tagged microtubules and showed that qua2 and tsd2 mutants have more random arrangements of microtubules. They also showed that the mutants are hypersensitive to mechanical stress and to oryzalin, a microtubule depolymerizing drug. This suggests that disruption of the integrity of the cell wall can feed back to alter microtubule patterning. Finally, they showed that QUA2 mutations reduce the expression of pectin and cellulose biosynthetic genes as well as genes involved in microtubule stability and cell wall integrity.
These results not only add to our knowledge of pectin biosynthesis, but also show how cell wall components interact to regulate cell wall properties and control growth and development. Future work promises to understand exactly how these interactions underlie the diverse functions of plant cell walls.
Chris Whitewoods
Department of Cell and Developmental Biology
John Innes Centre, Norwich
ORCID: 0000-0001-6886-3572
REFERENCES
Krupkova, E., Immerzeel, P., Pauly, M., and Schmulling, T. (2007). The TUMOROUS SHOOT DEVELOPMENT2 gene of Arabidopsis encoding a putative methyltransferase is required for cell adhesion and co-ordinated plant development. Plant J. 50: 735-750.
Mouille, G., Ralet, M.C., Cavelier, C., Eland, C., Effroy, D., Hematy, K., McCartney, L., Truong, H.N., Gaudon, V., Thibault, J.F., Marchant, A., and Hofte, H. (2007). Homogalacturonan synthesis in Arabidopsis thaliana requires a Golgi-localized protein with a putative methyltransferase domain. Plant J. 50: 605-614.