A Chloroplast-derived Signal Attenuates Growth in Red Light by Acting on the phyB-PIF Pathway
Author: David S. Favero1 (ORCID: 0000-0002-6879-0323)
Affiliation:
1RIKEN Center for Sustainable Resource Science, Yokohama, Kanagawa, 230-0045 Japan
Plant growth and development are regulated by a complex network of signals, many of which converge at the nucleus and affect gene transcription. Retrograde signals are transduced to the nucleus from organelles such as plastids and mitochondria to affect gene expression. Chloroplast retrograde signaling shapes the nuclear transcriptional profile in response to both endogenous developmental cues and external stimuli (Chan et al., 2016). Consistent with the observation that chloroplast activity is altered by some types of stress, retrograde signals are partially responsible for inducing gene expression changes in response to adverse environmental conditions. Methylerythritol cyclodiphosphate (MEcPP), a key metabolite in the plastidial isoprenoid biosynthetic pathway, is a retrograde signal. Both wounding and strong light promote accumulation of this metabolite, and MEcPP promotes the expression of stress-induced genes encoded in the nucleus (Xiao et al., 2012; Benn et al., 2016).
An Arabidopsis thaliana mutant that overaccumulates MEcPP, ceh1, has been useful for studying retrograde signals associated with this metabolite (Xiao et al., 2012). Enhanced accumulation of MEcPP in this mutant is caused by a defect in HYDROXYMETHYLBUTENYL DIPHOSPHATE SYNTHASE (HDS), a nuclear gene encoding the plastidial enzyme responsible for the reduction of MEcPP. The ceh1 mutant exhibits a constitutive stress response and substantially reduced growth (Fig. 1) (Xiao et al., 2012; Benn et al., 2016; Jiang et al., 2018; Jiang et al., 2019). However, although ceh1 contains a high level of salicylic acid (SA), a defense hormone that inhibits plant growth, SA does not cause the short hypocotyl phenotype observed in this mutant (Jiang et al., 2018). Rather, there is evidence that MEcPP attenuates growth in ceh1 partially by acting on phytochrome B (phyB), a photoreceptor that senses red/far-red light and negatively regulates hypocotyl elongation (Jiang et al., 2019). Loss-of-function mutations in PHYB partially suppress the reduced growth of ceh1 in white light, a condition under which MEcPP stabilizes phyB. However, gaps remain in our understanding of how this metabolite affects plant growth.
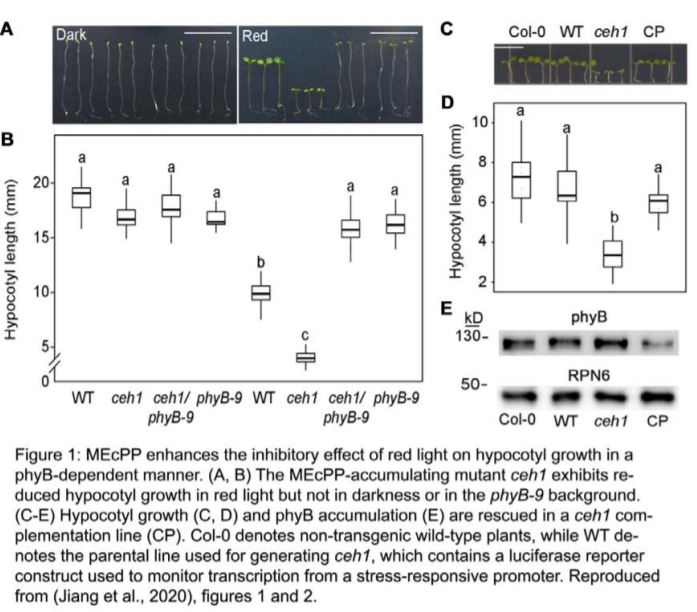 In this issue of Plant Physiology, Jiang et al. (2020) partially clarify the mechanism by which MEcPP represses growth. In particular, they demonstrate that this metabolite enhances the inhibitory effect of red light on hypocotyl elongation. The authors observed that while ceh1 seedlings elongate normally in darkness, growth of this mutant in either monochromatic red or blue light produces a clear reduction in hypocotyl growth (Fig. 1, A and B). This suggests that MEcPP enhances the repressive effects of both red and blue light signaling pathways on growth. Further, to clarify the role of phyB in MEcPP-mediated repression of growth in red and blue light, the authors generated a ceh1 phyB-9 double mutant. In blue light, ceh1 and ceh1 phyB-9 exhibited similar short-hypocotyl phenotypes, indicating that a phyB-independent mechanism is responsible for growth repression in this condition. By contrast, ceh1 completely fails to repress hypocotyl growth in ceh1 phyB-9 compared to phyB-9 in red light (Fig. 1, A and B). This suggests that the positive effect of MEcPP on phyB stability, initially reported in white light (Jiang et al., 2019), might be sufficient to explain how this metabolite enhances the repressive effect of red light on hypocotyl growth. Consistent with this idea, phyB accumulation is enhanced in ceh1 seedlings grown in red light (Fig. 1E), without a change in the level of PHYB transcripts (Jiang et al., 2020). Further support for this hypothesis is supplied by the observation that phyB protein accumulation in red light is rescued in a complementation line for ceh1 (CP), which exhibits similar hypocotyl growth to the wild type (Fig. 1, C–E).
In this issue of Plant Physiology, Jiang et al. (2020) partially clarify the mechanism by which MEcPP represses growth. In particular, they demonstrate that this metabolite enhances the inhibitory effect of red light on hypocotyl elongation. The authors observed that while ceh1 seedlings elongate normally in darkness, growth of this mutant in either monochromatic red or blue light produces a clear reduction in hypocotyl growth (Fig. 1, A and B). This suggests that MEcPP enhances the repressive effects of both red and blue light signaling pathways on growth. Further, to clarify the role of phyB in MEcPP-mediated repression of growth in red and blue light, the authors generated a ceh1 phyB-9 double mutant. In blue light, ceh1 and ceh1 phyB-9 exhibited similar short-hypocotyl phenotypes, indicating that a phyB-independent mechanism is responsible for growth repression in this condition. By contrast, ceh1 completely fails to repress hypocotyl growth in ceh1 phyB-9 compared to phyB-9 in red light (Fig. 1, A and B). This suggests that the positive effect of MEcPP on phyB stability, initially reported in white light (Jiang et al., 2019), might be sufficient to explain how this metabolite enhances the repressive effect of red light on hypocotyl growth. Consistent with this idea, phyB accumulation is enhanced in ceh1 seedlings grown in red light (Fig. 1E), without a change in the level of PHYB transcripts (Jiang et al., 2020). Further support for this hypothesis is supplied by the observation that phyB protein accumulation in red light is rescued in a complementation line for ceh1 (CP), which exhibits similar hypocotyl growth to the wild type (Fig. 1, C–E).
Also in this study, the authors demonstrated that MEcPP-mediated repression of hypocotyl growth in red light is dependent on PHYTOCHROME INTERACTING FACTORs (PIFs). The PIFs are key growth-promoting transcription factors, and importantly, they interact with phyB and are inhibited in multiple ways by this photoreceptor at the post-translational level (Favero, 2020). Therefore, enhanced accumulation of phyB in ceh1 may attenuate growth by reducing PIF activity. Consistent with this hypothesis, Jiang et al. (2020) found that the ceh1 mutant has no effect on hypocotyl growth in the pifq quadruple mutant, in which PIF1, PIF3, PIF4, and PIF5 are mutated. Conversely, overexpression of PIF4 or PIF5 in ceh1 attenuates the short hypocotyl phenotype of this mutant. It was further observed in this study that both PIF4 and PIF5 are expressed at lower levels in ceh1 grown in red light, suggesting that MEcPP additionally reduces PIF activity by inhibiting transcription of the genes that encode these transcription factors.
The findings in this study by Jiang et al. (2020) demonstrate that MEcPP attenuates growth in red light by promoting the accumulation of phyB and reducing PIF activity. Given that strong light and wounding both increase MEcPP accumulation, this retrograde signal may serve to attenuate growth in response to stress sensed by the chloroplast (Xiao et al., 2012). However, this hypothesis needs to be tested directly. Another unresolved question is how the MEcPP-derived signal is transduced to the nucleus and feeds into the phyB-PIF pathway. It is possible that increased accumulation of phyB in ceh1 is an indirect, downstream consequence of reduced PIF transcription in this mutant. Upon interaction with each other, PIF3 and phyB are co-ubiquitinated, and thereby both targeted for degradation (Ni et al., 2013; Ni et al., 2014). Additionally, other PIFs, including PIF4 and PIF5, also negatively regulate phyB accumulation (Leivar et al., 2012; Jiang et al., 2019). Therefore, it is possible that the MEcPP-derived signal acts more directly on PIF4 and PIF5 than on phyB, with reduced PIF transcription in ceh1 subsequently leading to less PIF-mediated degradation of the photoreceptor. Resolving this question of how the MEcPP signal is transduced to the phyB-PIF pathway is critical for better understanding how retrograde signaling affects plant growth.
LITERATURE CITED
Benn G, Bjornson M, Ke H, De Souza A, Balmond EI, Shaw JT, Dehesh K (2016) Plastidial metabolite MEcPP induces a transcriptionally centered stress-response hub via the transcription factor CAMTA3. Proc Natl Acad Sci U S A 113: 8855-8860
Chan KX, Phua SY, Crisp P, McQuinn R, Pogson BJ (2016) Learning the Languages of the Chloroplast: Retrograde Signaling and Beyond. Annu Rev Plant Biol 67: 25-53
Favero DS (2020) Mechanisms regulating PIF transcription factor activity at the protein level. Physiol Plant Published online February 14, 2020. https://doi.org/10.1111/ppl.13075
Jiang J, Rodriguez-Furlan C, Wang JZ, de Souza A, Ke H, Pasternak T, Lasok H, Ditengou FA, Palme K, Dehesh K (2018) Interplay of the two ancient metabolites auxin and MEcPP regulates adaptive growth. Nat Commun 9: 2262
Jiang J, Zeng L, Ke H, De La Cruz B, Dehesh K (2019) Orthogonal regulation of phytochrome B abundance by stress-specific plastidial retrograde signaling metabolite. Nat Commun 10: 2904
Leivar P, Monte E, Cohn MM, Quail PH (2012) Phytochrome signaling in green Arabidopsis seedlings: impact assessment of a mutually negative phyB-PIF feedback loop. Mol Plant 5: 734-749
Ni W, Xu SL, Chalkley RJ, Pham TN, Guan S, Maltby DA, Burlingame AL, Wang ZY, Quail PH (2013) Multisite light-induced phosphorylation of the transcription factor PIF3 is necessary for both its rapid degradation and concomitant negative feedback modulation of photoreceptor phyB levels in Arabidopsis. Plant Cell 25: 2679-2698
Ni W, Xu SL, Tepperman JM, Stanley DJ, Maltby DA, Gross JD, Burlingame AL, Wang ZY, Quail PH (2014) A mutually assured destruction mechanism attenuates light signaling in Arabidopsis. Science 344: 1160-1164
Xiao Y, Savchenko T, Baidoo EE, Chehab WE, Hayden DM, Tolstikov V, Corwin JA, Kliebenstein DJ, Keasling JD, Dehesh K (2012) Retrograde signaling by the plastidial metabolite MEcPP regulates expression of nuclear stress-response genes. Cell 149: 1525-1535