Last month I spent a few days at the Max Planck Institute of Molecular Plant Physiology in Potsdam, where I gave a talk about career building for early career researchers. I’ve shared the slides and here I summarize the take-home messages and links.
Chance favors the prepared mind
By now you’ve heard that there are more post-docs than professorships (see The PhD Factory). Statistically, early-career researchers are more likely to move into a non-tenure track academic position, position in industry or non-academic job than to land a tenure-track position. How do you find your best career path and navigate it successfully?
Here, I provide some career-planning suggestions, focusing on broadening your experience and broadening your footprint.
Broadening your experience / developing your transferable skills
Take advantage of opportunities to develop your skills beyond the bench, but at the same time, be sure to consider how these opportunities will strengthen your CV. Before you say “yes” to a request for your time, make sure you know how it will appear on your CV. For example, if you help out at a Fascination of Plants Day event, don’t write “Helped out at public event”, instead write “Raised funds, managed volunteers, assessed risk, assessed impact, reported outcomes” etc. Whenever possible, get a title for your position, such as “Coordinator of volunteers” or “Resources manager”.
Volunteering and mentoring opportunities abound, including:
Volunteer at a Fascination of Plants Day event
Mentor an iGEM team
Mentor students through PlantingScience.org
Mentor an undergraduate researcher
Volunteer with ASPB at an outreach event such as the AAAS Family Science Days, NSTA, NABT, the US Science and Engineering Festival, or even the White House Easter Egg Roll, or volunteer as an ASPB ambassador.
You can also develop transferable skills through science communication. Several established bloggers are happy to have a guest post, or you can start your own blog; see more on blogging here. Science communication doesn’t have to mean writing; you can communicate by creating podcasts, videos, photography, illustrations and infographics too.
Broadening your network
Many job seekers find their jobs through word-of-mouth and informal networking, so it stands to reason that the bigger your network, the more opportunities you’ll learn about. Some networks just happen, like the network that spans your lab and department. Broadening your networks requires effort on your part. As an example, here’s an interesting article about managing your mentoring network.
network, the more opportunities you’ll learn about. Some networks just happen, like the network that spans your lab and department. Broadening your networks requires effort on your part. As an example, here’s an interesting article about managing your mentoring network.
These days, most networking happens online. If you still think that social media is only about pop stars, selfies, and cat videos, think again. I recommend Twitter as an excellent source for networking opportunities. Not only will you get to listen in to what the leaders of the field are talking about, you’ll also see plenty of job ads on Twitter.
Like it or not, you have a digital profile, and managing it is an important part of your job-hunting strategy. Many journals are requiring ORCIDs for their authors, and it’s a painless process to sign up for one. A Google Scholar profile is often a top hit when searching for a scientist, so be sure yours is accurate and up-to-date (set up your profile by clicking on My Citations at the top of the Google Scholar page). LinkedIn and ResearchGate are good networking sites also. Some institutions allow postdocs and graduate students to have a profile page (if yours doesn’t, you can ask them to change their policy), and the new Plantae digital ecosystem for plant science is a great place to customize your profile and meet other plant scientists.
Professional societies like ASPB are excellent for networking, getting advice about careers, finding volunteer opportunities, and as an ASPB member you and can apply for travel awards to the annual meeting. Larger meetings like PlantBio16 and SEB often have workshops designed to help postdocs and graduate students learn new skills and prepare for the job hunt.
How do you decide which is the best career path for you?
First, you need to think about all the different things you can do with your PhD. See Klaue (2015) and resources from the National Postdoc Organization for some suggestions. Next, you need to think hard about what it is that you’d like to do. Everyone has their own values and priorities. Questions you need to think about include: What do you really enjoy doing? How important is a high salary? How important is job stability? Where do you want to live? Do you like to teach / manage others / write? What are your personal goals and values?
Sarah Blackford has numerous resources on her Bioscience Careers blog and book to help you think about what you want to do. You can set up an Individual Development Plan at Science Careers to help you identify jobs that are a good fit for your personal needs and values. If you’re considering a career in industry, try to attend a Careers Expo to meet representatives of many different organizations all at one place (see the careers calendar on Sarah Blackford’s blog). Start reading job ads early, to see what kinds of skills are required for some of your dream jobs. Talk to everyone you meet about their job: how did they find it, what do they do, what are the best (and worst) aspects of that career? Seek out contacts and resources; finding the best job for you depends on your networking and planning.
Preparing for an academic job
Don’t be discouraged by statistics. Academic jobs are competitive but there are opportunities, yet preparing for an academic job requires preparation. Start reading job ads early in your postdoc, and keep your CV up-to-date and polished. You’ll find academic jobs advertised in Nature and Science, and also professional society pages including ASPB and Plantae. The Chronicle for Higher Education is a great source of ads for short-term teaching positions such as sabbatical-leave replacements and one-year contracts. Scientists who have some teaching experience are much more competitive for tenure-track jobs at teaching-oriented institutions.
When you apply for an academic position, it is important to write a cover letter that is tailored for the position advertised. The search committee’s first cut is usually to eliminate applications with generic letters. Use your cover letter to show that you are a good fit for the job advertised. Depending on the focus of the institution, the search committee will be looking for evidence that you are able to secure external funds (grants), able to conduct and publish high-quality research, and have promise and often some experience as a teacher. They’ll also be evaluating you as a possible colleague; are you a good “citizen” who will work cooperatively within the department to take care of the departmental business? Do your skills fill a gap in the department but also provide opportunities for collaborations with others in the department?
You’ll also need to provide a research statement, and usually a statement of teaching interests and / or teaching philosophy. Writing these documents is not easy, so get advice (some excellent advice can be found in the links below), start early, and get feedback on your drafts. Finally, be sure to provide all of your documents to your referees so that they don’t accidently contradict your key messages.
You might get invited for a phone or Skype chat, which can be informal or formal. Success can lead to an invitation for a campus visit and formal interview, which will usually involve a research seminar and also sometimes a teaching talk. Research the department thoroughly ahead of a visit and express knowledge of and interest in your future colleagues (many a promising candidate has been torpedoed for forgetting this important point). The visit will include a chat with a senior administrator, and eventually a discussion about your start-up needs. Check out ASPB’s Membership Committee’s workshop Negotiating for Success Before you Sign at PlantBio16 to help you get your best deal.
Open your mind to possibilities
The most satisfying work is work that suits you. The more you explore your career options, the more you’ll learn about yourself. Don’t try to be a square peg in a round hole; just because you dreamed of doing something when you were young or even last year doesn’t mean that that is the right job for you now. Your options are broad, as long as you take control of your job search and remember the insightful words of Louis Pasteur, “Dans les champs de l’observation le hasard ne favorise que les esprits préparés” (In the fields of observation chance favors only the prepared mind).
Do you have any advice for those navigating the job search? Job hunters, what are your questions?
Resources
General career resources
Science Careers Be sure to create your free Individual Development Plan and check out the selection of useful booklets
Naturejobs.com
ChronicleVitae (Advice, blogs, tools; Some resources require free membership)
American Society of Cell Biologists – Career Wizard
American Society of Cell Biologists – Career Publications
EuroScience Open Forum Manchester 2016 = good careers programme (July, Manchester)
Naturejobs Career Expo Dusseldorf November 2016
The Chronical of Higher Education (news, blogs, advice and jobs – some pages require subscription)
Career Planning for Research Bioscientists (Sarah Blackford)
Boscience Careers Career Choice worksheet
The Thesis Whisperer, The Research Whisperer
Taking ownership of your own mentoring
Career Planning for Research Bioscientists (Sarah Blackford)
Broadening your experience and expertise (“transferable skills”)
Read and follow blogs at Nature Jobs and Science Careers
Inventory your skills at Vitae
Council on Undergraduate Research (CUR): Mentoring undergraduate students
iGEM – http://2016.igem.org
Plantae.org
PlantingScience.org
Broadening your footprint (getting your name known)
How to reach a wider audience for your research (SciDevNet)
Not Networking: Building Relationships for Success (Video, ASCB)
Getting the word out (The Scientist)
Twitter for plant scientists (ASPB)
To tweet or not to tweet (Science – on Jonathan Eisen)
Getting a faculty position
Insights into a faculty search committee By Sean Eddy
Six tips for writing an effective teaching statement (Amer Chem Soc)
How to write a statement of teaching philosophy (Montell, G., Chronical of Higher Ed)
Writing a teaching statement (PDF) (Yale University, ASCB)
At the Helm: Leading Your Laboratory (Kathy Barker)
Making the Right Moves, A Practical Guide to Scientific Management (HHMI)
Academic Job Search (University of Michigan)
The Professor is In (consultant / blogger)
Academic career resources (UCSF)
Non-Principle Investigator careers
EuroScienceJobs.com
Graduate survey: uncertain future (21 Oct 2015 Nature Jobs) Where do PhDs end up?
Klaue, Y. and Kellogg, D. (2015). Get that next job—how to break out of the postdoc trap. Molecular Biology of the Cell. 26: 3700-3703.
Careers After Biological Sciences
Alternative Careers in Science: Leaving the Ivory Tower. C. Robbins-Roth (ed). (2006).
Rescuing biomedical research What has to happen so PhDs have more options than academia? Organized by Bruce Alberts, Marc Kirschner, Shirley Tilghman, Harold Varmus
Education: The PhD Factory (Nature News Feature)
The Open Notebook. The story behind the best science stories
Ed Yong. (2010). On the origin of science writers
Science Careers blog (Biomed Central)
 REFERENCES
REFERENCES

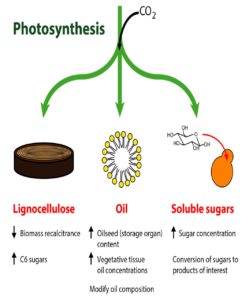 Shih et al. review how the many and diverse tools of plant synthetic biology can be applied towards bioenergy crops, focusing on traits related to lignocellulose, oil, and soluble sugars. Tools include those that edit genes, those that alter protein activities, and those that enable gene stacking in crop plants. Examples of successful manipulation of various metabolic pathways in bioenergy crops are provided, and areas where further tools and research are needed are highlighted. Plant J.
Shih et al. review how the many and diverse tools of plant synthetic biology can be applied towards bioenergy crops, focusing on traits related to lignocellulose, oil, and soluble sugars. Tools include those that edit genes, those that alter protein activities, and those that enable gene stacking in crop plants. Examples of successful manipulation of various metabolic pathways in bioenergy crops are provided, and areas where further tools and research are needed are highlighted. Plant J.  REFERENCES
REFERENCES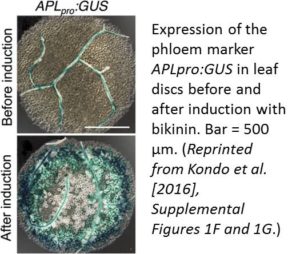 Investigating how plants grow and develop often requires a bit of creativity. For example, deep within the plant, the vascular cambium, a layer of embryonic, highly cytoplasmic cells, gives rise to xylem and phloem tissue, which must expand throughout the plant’s lifetime. Water and nutrients primarily flow through the xylem, whereas photosynthate and signaling molecules travel through the phloem. While anatomical and labeling studies have shed light on the sequential development of vascular tissue, this process is notoriously difficult to track due to the limited accessibility of the plant vasculature. However, under the proper conditions, various cells can be coaxed into producing vascular cells in culture, which greatly facilitates their investigation. Such analyses have revealed many key factors that regulate the differentiation of xylem cells from vascular cambium cells (reviewed in
Investigating how plants grow and develop often requires a bit of creativity. For example, deep within the plant, the vascular cambium, a layer of embryonic, highly cytoplasmic cells, gives rise to xylem and phloem tissue, which must expand throughout the plant’s lifetime. Water and nutrients primarily flow through the xylem, whereas photosynthate and signaling molecules travel through the phloem. While anatomical and labeling studies have shed light on the sequential development of vascular tissue, this process is notoriously difficult to track due to the limited accessibility of the plant vasculature. However, under the proper conditions, various cells can be coaxed into producing vascular cells in culture, which greatly facilitates their investigation. Such analyses have revealed many key factors that regulate the differentiation of xylem cells from vascular cambium cells (reviewed in 



 Nemhauser and Torii define synthetic biology as “an engineering approach to design, build and analyize dynamic molecular devices and/or pathways from biological components to produce cells and organisms with customized functionality.” In their review, they describe several plant synthetic biology approaches and outcomes, including efforts to identify and remediate toxins, engineer receptors, modify signals and molecular motars, and reprogram epigenetic controls. Nature Plants
Nemhauser and Torii define synthetic biology as “an engineering approach to design, build and analyize dynamic molecular devices and/or pathways from biological components to produce cells and organisms with customized functionality.” In their review, they describe several plant synthetic biology approaches and outcomes, including efforts to identify and remediate toxins, engineer receptors, modify signals and molecular motars, and reprogram epigenetic controls. Nature Plants 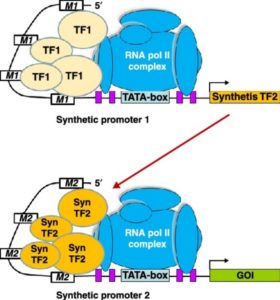 Many plant traits are multigenic, so engineering them requires modulating the expression of several genes simultaneously. Synthetic promoters and transcription factors offer such a possibility. For example, a cis-element can be introduced into the promoter of each gene of interest, and a synthetic transcription factor that activates gene expression via this cis-element and is itself activatable can be introduced into the cells. Liu and Stewart review the current status and trends, and future applications, of plant synthetic promoters and transcription factors. Curr. Opin. Biotech.
Many plant traits are multigenic, so engineering them requires modulating the expression of several genes simultaneously. Synthetic promoters and transcription factors offer such a possibility. For example, a cis-element can be introduced into the promoter of each gene of interest, and a synthetic transcription factor that activates gene expression via this cis-element and is itself activatable can be introduced into the cells. Liu and Stewart review the current status and trends, and future applications, of plant synthetic promoters and transcription factors. Curr. Opin. Biotech. 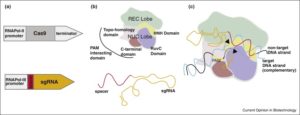 Raitskin and Patron review efforts to express multiple single guide RNA (sgRNAs) and Cas9 in plants for the coordinated expression of many genes. They argue for the need to create single plasmids carrying the sgRNAs and Cas9, using a Type IIS restriction endonuclease-mediated assembly method. Curr. Opin. Biotech.
Raitskin and Patron review efforts to express multiple single guide RNA (sgRNAs) and Cas9 in plants for the coordinated expression of many genes. They argue for the need to create single plasmids carrying the sgRNAs and Cas9, using a Type IIS restriction endonuclease-mediated assembly method. Curr. Opin. Biotech.