Monitoring the Dynamics of Freezing in Trees
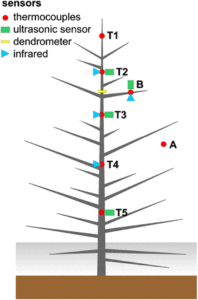 Ice formation within plants influences their physiology mechanically, hydraulically, and at a cellular level. Mechanical strain occurs as water expands during freezing and tension is induced in the remaining liquid-phase sap. Xylem cavitation is initiated upon freezing due to the low (i.e. negative) water potential of ice and the low solubility of gases in ice. Embolisms subsequently develop upon thawing when gas bubbles released from the ice coalesce, expand, and block the complete conduit. The low water potential of ice also generates an osmotic disturbance, which can lead to membrane breakage and cellular lysis. When temperatures drop below 0°C, the xylem sap remains in a metastable, supercooled state until crystallization is initiated around a nucleus. But do all parts of the tree freeze at more or less the same time? A contribution by Charrier et al. () focuses on ice nucleation and propagation, related water shifts, and xylem cavitation as well as cell damage. To examine these processes, the authors employed nondestructive techniques such as the in situ monitoring of xylem (thermocouples) and surface temperatures (infrared imaging), ultrasonic emissions, and dendrometer analysis. Field experiments during late winter on Picea abies growing at the alpine timberline revealed three distinct freezing patterns: (1) from the top of the tree toward the base, (2) from thin branches toward the main stem’s top and base, and (3) from the base toward the top. Infrared imaging showed freezing within branches from their base toward distal parts. Such complex freezing patterns cause dynamic and heterogeneous patterns in water potential and probably in cavitation. Thus, the freezing process in trees is more complicated than previously believed.
Ice formation within plants influences their physiology mechanically, hydraulically, and at a cellular level. Mechanical strain occurs as water expands during freezing and tension is induced in the remaining liquid-phase sap. Xylem cavitation is initiated upon freezing due to the low (i.e. negative) water potential of ice and the low solubility of gases in ice. Embolisms subsequently develop upon thawing when gas bubbles released from the ice coalesce, expand, and block the complete conduit. The low water potential of ice also generates an osmotic disturbance, which can lead to membrane breakage and cellular lysis. When temperatures drop below 0°C, the xylem sap remains in a metastable, supercooled state until crystallization is initiated around a nucleus. But do all parts of the tree freeze at more or less the same time? A contribution by Charrier et al. () focuses on ice nucleation and propagation, related water shifts, and xylem cavitation as well as cell damage. To examine these processes, the authors employed nondestructive techniques such as the in situ monitoring of xylem (thermocouples) and surface temperatures (infrared imaging), ultrasonic emissions, and dendrometer analysis. Field experiments during late winter on Picea abies growing at the alpine timberline revealed three distinct freezing patterns: (1) from the top of the tree toward the base, (2) from thin branches toward the main stem’s top and base, and (3) from the base toward the top. Infrared imaging showed freezing within branches from their base toward distal parts. Such complex freezing patterns cause dynamic and heterogeneous patterns in water potential and probably in cavitation. Thus, the freezing process in trees is more complicated than previously believed.





Leave a Reply
Want to join the discussion?Feel free to contribute!