Insights into the Trans-Golgi Network and Protein Secretion
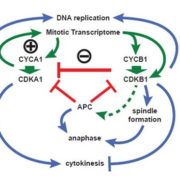
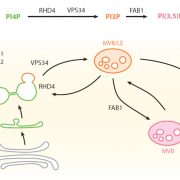
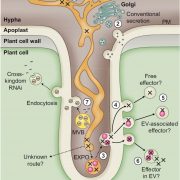
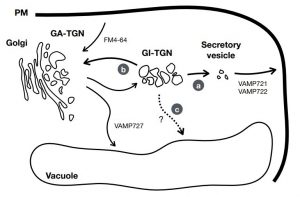 In eukaryotic cells, the movement of cargo between single membrane-bound organelles such as the endoplasmic reticulum (ER), Golgi apparatus, trans-Golgi network (TGN), endosomes, lysosomes, and vacuoles is mediated by membrane trafficking. At the donor organelle, cargo molecules are loaded into transport vesicles, which then become tethered to and fuse with the target organelle membrane to discharge and deliver their cargos to a destination compartment. The coordination required for membrane fusion events and for the delivery of cargos to their correct destinations is secured by specific interactions between members of the SNARE (soluble N-ethyl-maleimide sensitive factor attachment protein receptor) family proteins. The post-Golgi interface, including the TGN, is a pivotal hub for two major trafficking pathways. Golgi-associated TGNs are adjacent to and function jointly with the Golgi apparatus. On the other hand, free TGNs, which are cellular components described only in plant cells, originate from Golgi-associated-TGNs but are thought to operate independently from the Golgi apparatus and GA-TGNs. Uemura et al. (10.1104/pp.18.01228) now show that free TGNs are associated with two SNARE proteins, VESICLE-ASSOCIATED MEMBRANE PROTEIN 721 (VAMP721) and VAMP722, which are known to be responsible for protein secretion and for extracellular defense. The authors report that the perturbations of Golgi-associated- and free-TGN integrity induced by the syp42syp43 double mutation caused impaired transport of VAMP721 to the plasma membrane, thereby directly connecting free TGNs to secretory pathway components. In addition, quantitative proteomic analyses of leaf apoplastic fluid revealed that SYP4 and VAMP721 are needed for the constitutive and pathogen-inducible secretion of cell wall-modification enzymes that are important for plant growth and extracellular defense.
In eukaryotic cells, the movement of cargo between single membrane-bound organelles such as the endoplasmic reticulum (ER), Golgi apparatus, trans-Golgi network (TGN), endosomes, lysosomes, and vacuoles is mediated by membrane trafficking. At the donor organelle, cargo molecules are loaded into transport vesicles, which then become tethered to and fuse with the target organelle membrane to discharge and deliver their cargos to a destination compartment. The coordination required for membrane fusion events and for the delivery of cargos to their correct destinations is secured by specific interactions between members of the SNARE (soluble N-ethyl-maleimide sensitive factor attachment protein receptor) family proteins. The post-Golgi interface, including the TGN, is a pivotal hub for two major trafficking pathways. Golgi-associated TGNs are adjacent to and function jointly with the Golgi apparatus. On the other hand, free TGNs, which are cellular components described only in plant cells, originate from Golgi-associated-TGNs but are thought to operate independently from the Golgi apparatus and GA-TGNs. Uemura et al. (10.1104/pp.18.01228) now show that free TGNs are associated with two SNARE proteins, VESICLE-ASSOCIATED MEMBRANE PROTEIN 721 (VAMP721) and VAMP722, which are known to be responsible for protein secretion and for extracellular defense. The authors report that the perturbations of Golgi-associated- and free-TGN integrity induced by the syp42syp43 double mutation caused impaired transport of VAMP721 to the plasma membrane, thereby directly connecting free TGNs to secretory pathway components. In addition, quantitative proteomic analyses of leaf apoplastic fluid revealed that SYP4 and VAMP721 are needed for the constitutive and pathogen-inducible secretion of cell wall-modification enzymes that are important for plant growth and extracellular defense.