Dual regulation of Arabidopsis AGO2 by arginine methylation (Nature Comms)
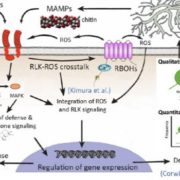
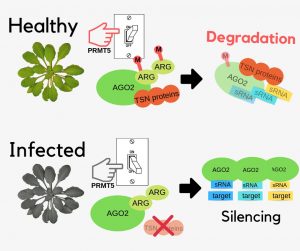 Plants have natural mechanisms against pathogen infections. Post-translational modifications (PTMs) on key proteins involved in RNAi pathways are needed to control these immune responses. Argonaute (AGO) proteins are targets of PTMs to direct the silencing of genes. Here, Hu et al. identified the role of PRMT5, an arginine methyltransferase, in directing symmetric dimethylation in the N-terminal of Arabidopsis AGO2 by using coimmunoprecipitation coupled with mass spectroscopy analysis and bimolecular fluorescence complementation in mutants. This methylation leads to AGO2 degradation by 26S proteasome. Meanwhile, the methylarginine residues interacts with Tudor-domain proteins (TSNs) which also have degradation activity controlling abundance of AGO2 associated small RNAs. With these results, the authors suggest that in bacterial infected plants, PRMT5 is downregulated increasing the levels of AGO2 and its bound sRNAs and contributing to increased host immunity. Further studies of post-translational modifications on RNAi pathways may be useful for the development of pathogen-resistant crop varieties. (Summary by Ana Valladares) Nature Comms s41467-019-08787-w
Plants have natural mechanisms against pathogen infections. Post-translational modifications (PTMs) on key proteins involved in RNAi pathways are needed to control these immune responses. Argonaute (AGO) proteins are targets of PTMs to direct the silencing of genes. Here, Hu et al. identified the role of PRMT5, an arginine methyltransferase, in directing symmetric dimethylation in the N-terminal of Arabidopsis AGO2 by using coimmunoprecipitation coupled with mass spectroscopy analysis and bimolecular fluorescence complementation in mutants. This methylation leads to AGO2 degradation by 26S proteasome. Meanwhile, the methylarginine residues interacts with Tudor-domain proteins (TSNs) which also have degradation activity controlling abundance of AGO2 associated small RNAs. With these results, the authors suggest that in bacterial infected plants, PRMT5 is downregulated increasing the levels of AGO2 and its bound sRNAs and contributing to increased host immunity. Further studies of post-translational modifications on RNAi pathways may be useful for the development of pathogen-resistant crop varieties. (Summary by Ana Valladares) Nature Comms s41467-019-08787-w