Photosynthetic Oxygen Production: New Method Brings to Light Forgotten Flux
Oxygen (O2) is evolved during photosynthetic electron transport when water is split by the oxygen-evolving complex to provide protons and electrons to the chloroplastic electron chain, thereby generating ATP and NADPH—the energy source and reducing power for plant metabolism. The majority of this chemical energy is used to drive photosynthetic carbon metabolism, which consists of ribulose-1,5-bisphosphate carboxylation (photosynthetic carbon reduction cycle) and oxygenation (photosynthetic carbon oxidation cycle); with a combined electron requirement = JA. Four electrons are required for every O2 evolved so that gross O2 production (GOP) is related to linear electron transport (J) according to J/4. When linear electron transport is used only to drive CO2 fixation, the consumption of O2 and the release of CO2 by photosynthetic carbon oxidation and mitochondrial respiration is such that net O2 production (NOP) is equal to net CO2 assimilation (Anet; provided the respiratory quotient is 1, but see Tcherkez et al., 2017).
Additionally, electrons can be used for alternative noncyclic electron transport (ANCET), including, for example, the photoreduction of O2 itself forming reactive oxygen species (Mehler-peroxidase reactions or “water-water cycle”; Asada, 1999), chloroplastic anabolism (e.g. lipids; Stumpf et al., 1963), the reduction of oxaloacetate to malate (which is exported to the mitochondria; Scheibe, 2004), and nitrogen assimilation (Bloom et al., 1989). ANCET has been hypothesized both as a way to regulate ATP/NADPH ratio to meet the changing energy demands of cellular metabolism and as a mechanism to prevent photodamage through utilizing excess reductant when the photon flux density exceeds the energy requirement of CO2 fixation (e.g. under high irradiance, cold temperatures, water stress closing stomata; e.g. Badger, 1985; Ort and Baker, 2002; Robinson, 1988). Importantly, there is no formal evidence for how electron flows interact, particularly under fluctuating light conditions (Morales et al., 2018).
As ANCET allows for greater rates of linear electron transport to be sustained, total electron transport (Jt) will be greater than JA. Conversely, the effect on O2 uptake will be dependent on the metabolic pathway involved. For example, in the Mehler-peroxidase reactions, there is no net change in O2 so that NOP will remain equal to Anet. But in the reduction of nitrate, the ratio between N-linked O2 production and O2 consumption is highly dependent on the amino acid synthesized (Noctor and Foyer, 1998). In this case, NOP will not always equal Anet because O2 and CO2 may not be balanced in metabolism (Skillman, 2008). Consequently, concomitant measurements of CO2 and O2 fluxes are important to the understanding of how plants regulate the use of light energy, with different fates having very different metabolic outcomes.
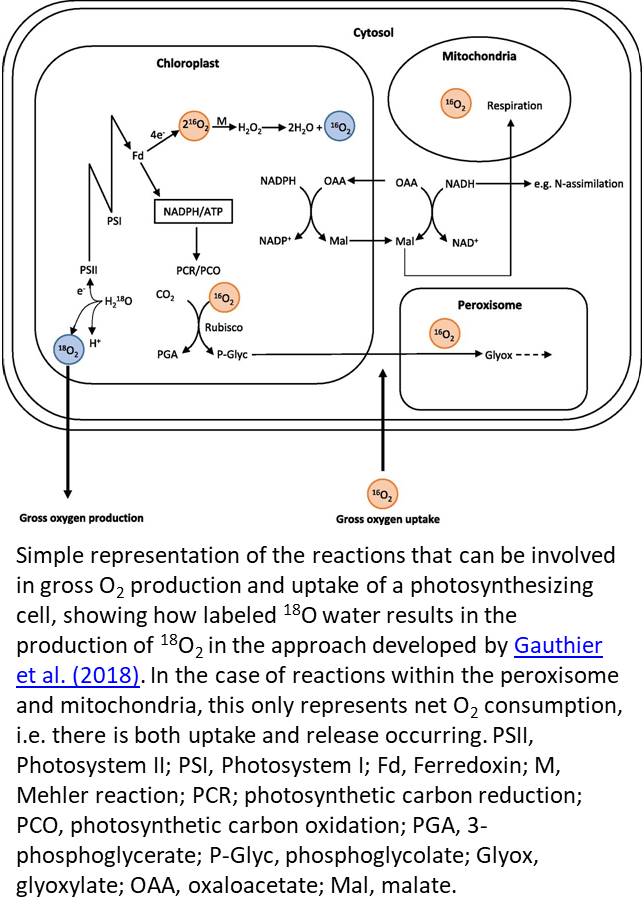
The earliest measurements of O2 evolution were unable to distinguish GOP from uptake of O2 (Hill, 1937). The mass spectrometry method established by Mehler and Brown (1952) solved this problem by employing O2 isotope tracers to independently monitor fluxes of 16O2 and 18O2. In this method, pure 18O2 was supplied to the gas headspace of a closed chamber, and the decline in 18O2 was attributed to O2 uptake. O2 evolved carries the same isotopic composition as the water from which it is generated; in this case, the dominant isotope in the water was 16O (Fig. 1). The 18O-labeling approach was further applied to leaf disks (e.g. Tourneux and Peltier, 1995), whole excised leaves (e.g. Volk and Jackson, 1972), and entire plants (Gerbaud and André, 1980), illuminating the fate of O2 in vivo.
The limitation of closed gas exchange systems is that measurements can only be undertaken for short periods of time (seconds to minutes) before the CO2 concentration is depleted. Consequently, CO2:O2 is not constant, which changes the relative rates of carboxylation and oxygenation so that estimates of GOP and O2 uptake will be inaccurate. This limitation was overcome in the mass spectrometry approach by replacing CO2 consumed through periodic influx of CO2 into the chamber, allowing for steady-state quantification and extending the ability to measure O2 fluxes under a range of conditions and physiological states (Canvin et al., 1980). At the same time, advances were being made in the use of chlorophyll fluorescence, which provides information on PSII quantum yield (Baker, 2008). Genty et al. (1989) provided the empirical link between fluorescence and electron transport rate, replacing the need to directly measure O2 evolution. Chlorophyll fluorescence is now one of the most popular techniques in plant physiology because of its ease of use and relatively low cost. This has been aided by the capacity to multiplex fluorescence measurements with H2O and CO2 gas exchange in portable, commercially available instruments, opening up the possibility of measuring plant function outside of the laboratory. Consequently, in vivo measurements of O2 fluxes have substantially declined over the last 20 years.
In this issue of Plant Physiology, Gauthier et al. (2018) remind us why it is so important to return our attention to O2, providing us with a new, elegant open-path system to measure O2 fluxes. Their method is a “reverse” isotopic approach, involving 18O-labeling of leaf water rather than the air so that the isotopic composition of O2 that is evolved during water splitting has a signature very different to that of ambient O2 (Fig. 1). The use of considerable 18O enrichment is imperative since the contribution of NOP in a background of 21% O2 is likely to be in the order of 0.05% (e.g. 100 μmol mol−1 NOP/210,000 μmol mol−1 ambient O2), making it difficult ordinarily to accurately detect a change in δ18O of O2 associated with NOP in the air surrounding the leaf.
The method remains highly technical, requiring the use of three high-precision instruments. The isotopic composition and concentration of CO2 and H2O vapor are measured by laser spectroscopy, and the δ18O2 and δO2/N2 (to estimate O2 concentration) by mass spectrometry. A custom-made chamber is also required to house the excised leaf and its 18O-labeled water source, which helps to prevent leaks across the gaskets from around the petiole. Importantly, the open gas exchange system improves the ability to achieve steady-state measurements, and labeling water versus the use of pure 18O2 gas solves the affordability issue, which has greatly limited the adoption of open systems.
While chlorophyll fluorescence has become the popular option for measuring electron transport rate, it is not without assumptions. For example, it is frequently assumed that leaves absorb 84% of incident photons and that 50% of these photons are absorbed by PSII; however, this may not always be the case (Baker, 2008). This may lead to an overestimate of electron transport rate when computed from fluorescence compared with measurements of GOP. Furthermore, accurate determination of JA is particularly relevant for the estimation of mesophyll conductance, which was one application highlighted by Gauthier et al. (2018). The Mehler-peroxidase reactions, which have been shown to range from 0% to 30% (Driever and Baker, 2011), would lead to an overestimate of electron fluxes associated with the photosynthetic carbon reduction/oxygenation cycles in both methods. However, the advantage of the isotope labeling approach is that the contribution of the Mehler reaction to gross O2 production can be quantified by coupling measurements of GOP with NOP (e.g. Furbank et al., 1982; see Fig. 1). Now that we have a renewed ability to measure O2 fluxes, these assumptions should not be ignored.
Besides understanding the trade-off between efficiency and photoprotection for improved agricultural production (Murchie and Niyogi, 2011), the different electron fates have important implications for understanding global O2 fluxes. Notably, O2 uptake associated with photorespiration, mitochondrial respiration, and the Mehler-peroxidase reactions have different isotope fractionation factors (Guy et al., 1993) so that the quantification of individual pathway fluxes is needed to constrain estimates of global primary production from δ18O information (Welp et al., 2011).
It is high time we revisited the measurement of O2 fluxes, and the new method developed by Gauthier et al. (2018) provides us with the necessary capacity to do so.
REFERENCES
Asada K (1999) The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50: 601–639
Badger MR (1985) Photosynthetic oxygen exchange. Annu Rev Plant Physiol 36: 27–53
Baker NR (2008) Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu Rev Plant Biol 59: 89–113
Bloom AJ, Caldwell RM, Finazzo J, Warner RL, Weissbart J (1989) Oxygen and carbon dioxide fluxes from barley shoots depend on nitrate assimilation. Plant Physiol 91: 352–356
Canvin DT, Berry JA, Badger MR, Fock H, Osmond CB (1980) Oxygen exchange in leaves in the light. Plant Physiol 66: 302–307
Driever SM, Baker NR (2011) The water-water cycle in leaves is not a major alternative electron sink for dissipation of excess excitation energy when CO2 assimilation is restricted. Plant Cell Environ 34: 837–846
Furbank RT, Badger MR, Osmond CB (1982) Photosynthetic oxygen exchange in isolated cells and chloroplasts of C3 plants. Plant Physiol 70: 927–931
Gauthier PPG, Battle MO, Griffin KL, Bender ML (2018) Measurement of gross photosynthesis, respiration in the light, and mesophyll conductance using H218O labeling. Plant Physiol 177: 62–74
Genty B, Briantais J-M, Baker NR (1989) The relationship between the quantum yield of photosynthetic
Gerbaud A, André M (1980) Effect of CO2, O2, and light on photosynthesis and photorespiration in wheat. Plant Physiol 66: 1032–1036
Guy RD, Fogel ML, Berry JA (1993) Photosynthetic fractionation of the stable isotopes of oxygen and carbon. Plant Physiol 101: 37–47
Hill R (1937) Oxygen evolved by isolated chloroplasts. Nature 139: 881–882
Mehler AH, Brown AH (1952) Studies on reactions of illuminated chloroplasts. III. Simultaneous photoproduction and consumption of oxygen studied with oxygen isotopes. Arch Biochem Biophys 38: 365–370
Morales A, Yin X, Harbinson J, Driever SM, Molenaar J, Kramer DM, Struik PC (2018) In silico analysis of the regulation of the photosynthetic electron transport chain in C3 plants. Plant Physiol 176: 1247–1261
Murchie EH, Niyogi KK (2011) Manipulation of photoprotection to improve plant photosynthesis. Plant Physiol 155: 86–92
Noctor G, Foyer CH (1998) A re-evaluation of the ATP:NADPH budget during C3 photosynthesis: a contribution from nitrate assimilation and its associated respiratory activity? J Exp Bot 49: 1895–1908
Ort DR, Baker NR (2002) A photoprotective role for O2 as an alternative electron sink in photosynthesis? Curr Opin Plant Biol 5: 193–198
Robinson JM (1988) Does O2 photoreduction occur within chloroplasts in vivo? Physiol Plant 72: 666–680
Scheibe R (2004) Malate valves to balance cellular energy supply. Physiol Plant 120: 21–26
Skillman JB (2008) Quantum yield variation across the three pathways of photosynthesis: not yet out of the dark. J Exp Bot 59: 1647–1661
Stumpf PK, Bové JM, Goffeau A (1963) Fat metabolism in higher plants. XX. Relation of fatty acid synthesis and photophosphorylation in lettuce chloroplast. Biochim Biophys Acta 70: 260–270
Tcherkez G, Gauthier P, Buckley TN, Busch FA, Barbour MM, Bruhn D, Heskel MA, Gong XY, Crous KY, Griffin K, et al. (2017) Leaf day respiration: low CO2 flux but high significance for metabolism and carbon balance. New Phytol 216: 986–1001
Tourneux C, Peltier G (1995) Effect of water deficit on photosynthetic oxygen exchange measured using 18O2 and mass spectrometry in Solanum tuberosum L. leaf discs. Planta 195: 570–577
Volk RJ, Jackson WA (1972) Photorespiratory phenomena in maize: oxygen uptake, isotope discrimination, and carbon dioxide efflux. Plant Physiol 49: 218–223
Welp LR, Keeling RF, Meijer HAJ, Bollenbacher AF, Piper SC, Yoshimura K, Francey RJ, Allison CE, Wahlen M (2011) Interannual variability in the oxygen isotopes of atmospheric CO2 driven by El Niño. Nature 477: 579–582