UBIQUITIN-SPECIFIC PROTEASE (UBP14) interacts with HY5 to promote photomorphogenesis under dark-to-light conditions
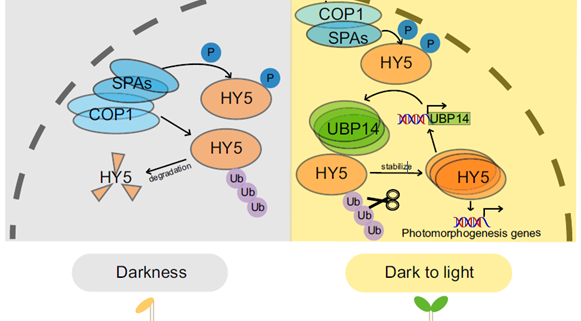
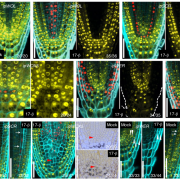
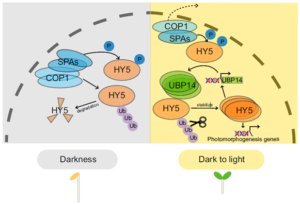 ELONGATED HYPOCOTYL5 (HY5) is a transcription factor that regulates about one-third of Arabidopsis genes, affecting growth and development of seedlings through light and hormone signaling. UBIQUITIN-SPECIFIC PROTEASE (UBP14) is a deubiquitinating enzyme that removes ubiquitin from substrate proteins. In a new study, Fang et al. performed bi-molecular fluorescence complementation, and yeast two hybrid, pull down, and co-immunoprecipitation assays to validate the interaction of UBP14 and HY5 in vitro and in vivo. Their experiments show that UBP14 is involved in stabilizing the protein expression of HY5 under dark-to-light conditions, which is essential for photomorphogenesis (promoting cotyledon opening and inhibiting hypocotyl growth). On the contrary, the absence of UBP14 leads to a slower accumulation of HY5 protein. UBP14 preferentially stabilizes the non-phosphorylated form of HY5 which is more active in binding other downstream genes compared to the phosphorylated form. HY5 can directly bind to two G-box elements present near the transcription start site in the promoter of UBP14 and promote its positive feedback, making itself more stable under light conditions. This discovery provides an excellent example of how a deubiquitinating enzyme contributes to cotyledon opening. (Summary by Asif Ali @pbgasifkalas) PNAS. 10.1073/pnas.2404883121
ELONGATED HYPOCOTYL5 (HY5) is a transcription factor that regulates about one-third of Arabidopsis genes, affecting growth and development of seedlings through light and hormone signaling. UBIQUITIN-SPECIFIC PROTEASE (UBP14) is a deubiquitinating enzyme that removes ubiquitin from substrate proteins. In a new study, Fang et al. performed bi-molecular fluorescence complementation, and yeast two hybrid, pull down, and co-immunoprecipitation assays to validate the interaction of UBP14 and HY5 in vitro and in vivo. Their experiments show that UBP14 is involved in stabilizing the protein expression of HY5 under dark-to-light conditions, which is essential for photomorphogenesis (promoting cotyledon opening and inhibiting hypocotyl growth). On the contrary, the absence of UBP14 leads to a slower accumulation of HY5 protein. UBP14 preferentially stabilizes the non-phosphorylated form of HY5 which is more active in binding other downstream genes compared to the phosphorylated form. HY5 can directly bind to two G-box elements present near the transcription start site in the promoter of UBP14 and promote its positive feedback, making itself more stable under light conditions. This discovery provides an excellent example of how a deubiquitinating enzyme contributes to cotyledon opening. (Summary by Asif Ali @pbgasifkalas) PNAS. 10.1073/pnas.2404883121