To grow or not to grow: specific lipoxygenases control wound-induced growth restriction
To grow or not to grow: specific lipoxygenases control wound-induced growth restriction
Amna Mhamdi
Ghent University, Department of Plant Biotechnology and Bioinformatics, and VIB Center for Plant Systems Biology, 9052 Ghent, Belgium
Address correspondence to: [email protected]
As most vegetable growers know, plants have a lot of predators. Repetitive wounding events caused by herbivorous insects and necrotrophic pathogens often lead to tissue damage and growth restriction. When Arabidopsis (Arabidopsis thaliana) is subjected to recurrent wounding, leaf growth is dramatically reduced. In nature, similar stunted phenotypes can be observed in ornamental bonsai plants cultivated by skilled gardeners or when plants are attacked by herbivorous mammals or insects. In these contexts, plants overproduce jasmonates, of which the best known is jasmonic acid (JA) (Browse, 2009). Inhibition of vegetative growth, as part of the wound response, occurs in wild type plants but not in JA mutants (Zhang and Turner, 2008). Jasmonates are also required for reproductive growth as impairment in JA synthesis or perception results in male sterility (Stintzi and Browse, 2000; Park et al., 2002).
JA synthesis is initiated in plastids where 13-lipoxygenases (LOX) add oxygen to the polyunsaturated fatty acids linolenic acid (18:3) and hexadecatrienoic acid (16:3) to form the corresponding 13-hydroperoxides which are then cyclized to 12-oxo-phytodienoic acid, the JA precursor. In Arabidopsis leaves, JA biosynthesis depends on four specific 13-LOXs (LOX2, 3, 4 and 6). Their roles in reproductive development have been characterized; however, the function of each of these enzymes in controlling vegetative growth remains elusive.
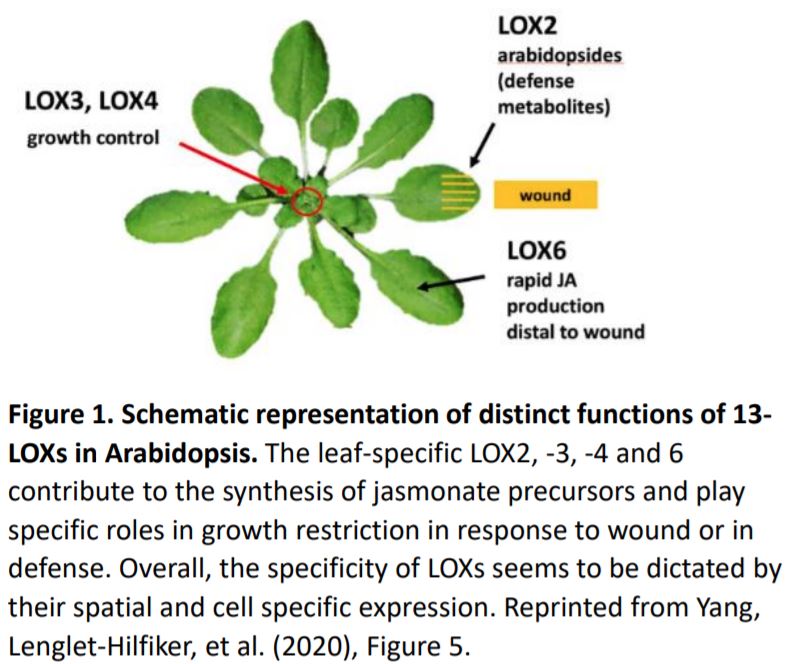 In this issue of Plant Physiology, Yang, Lenglet-Hilfiker, et al. (2020) used a genetic approach to elucidate the contribution of each LOX to Arabidopsis rosette growth restriction in response to wounding. Analysis of plants with loss-of-function lox alleles subjected to serial wounding assays showed that LOX3 and to a lesser extent LOX4 produce the majority of JA precursors necessary for wound-induced rosette growth restriction. The authors exploited the fatty acid oxygenation upregulated 2 (fou2) genetic background that over-accumulates JA, which in turn triggers a transcriptomic signature similar to that produced by chewing insects (Bonaventure et al., 2007a; 2007b). The fou2 mutant harbors a mutation in the putative voltage sensor of the TWO PORE CHANNEL 1 gene (TPC1), which encodes a Ca2+-permeant non-selective cation channel. Yang, Lenglet-Hilfiker, et al. found that the severe JA-induced growth inhibition in fou2 is largely suppressed by mutations in LOX3 and LOX4. Collectively, these data reveal the roles of LOX3 and LOX4 in rosette growth and suggest, strikingly, that the stunted and bonsai-like wound phenotypes could be recapitulated through genetic activation of 13-LOXs in the absence of wounding (Figure 1).
In this issue of Plant Physiology, Yang, Lenglet-Hilfiker, et al. (2020) used a genetic approach to elucidate the contribution of each LOX to Arabidopsis rosette growth restriction in response to wounding. Analysis of plants with loss-of-function lox alleles subjected to serial wounding assays showed that LOX3 and to a lesser extent LOX4 produce the majority of JA precursors necessary for wound-induced rosette growth restriction. The authors exploited the fatty acid oxygenation upregulated 2 (fou2) genetic background that over-accumulates JA, which in turn triggers a transcriptomic signature similar to that produced by chewing insects (Bonaventure et al., 2007a; 2007b). The fou2 mutant harbors a mutation in the putative voltage sensor of the TWO PORE CHANNEL 1 gene (TPC1), which encodes a Ca2+-permeant non-selective cation channel. Yang, Lenglet-Hilfiker, et al. found that the severe JA-induced growth inhibition in fou2 is largely suppressed by mutations in LOX3 and LOX4. Collectively, these data reveal the roles of LOX3 and LOX4 in rosette growth and suggest, strikingly, that the stunted and bonsai-like wound phenotypes could be recapitulated through genetic activation of 13-LOXs in the absence of wounding (Figure 1).
Further, to test if the bonsai-like phenotype could be genetically phenocopied in unwounded tissues, Yang, Lenglet-Hilfiker, et al. investigated the relevance of LOX3 activation in phloem tissue since LOX3 promoter activity spans the xylem and phloem and both these tissues are known to play major roles in leaf-to-leaf signaling (Chauvin et al., 2016; Nguyen et al., 2018). To accomplish this, Yang, Lenglet-Hilfiker, and coauthors designed a novel and elegant system where they expressed different variants of the TCP1 protein, under a phloem specific promoter, and introduced it in the lox quadruple and triple mutant backgrounds with and without a functional LOX3. Phenotypic and biochemical analyses revealed that expressing a hyperactive version of TCP1 genetically activates LOX3-dependent JA production and strongly constrains rosette growth in the absence of wounding (Figure 1).
Therefore, this new study by Yang, Lenglet-Hilfiker, et al. reveals how plants suffer the slings and arrows of herbivory and decide to grow or, more often, not to grow, defining specific functions for LOX3- and phloem-derived signals and providing an exciting genetic tool that will be valuable to further mechanistically elucidate the wound response and cell signaling during specific stresses.
References
Bonaventure G, Gfeller A, Proebsting WM, Hoerstensteiner S, Chételat A, Martinoia E, Farmer EE. (2007a) A gain of function allele of TPC1 activates oxylipin biogenesis after leaf wounding in Arabidopsis. Plant J. 49, 889-898.
Bonaventure G, Gfeller A, Rodríguez VM, Armand F, Farmer EE. (2007b) The fou2 gain-of-function allele and the wild-type allele of Two Pore Channel 1 contribute to different extents or by different mechanisms to defense gene expression in Arabidopsis. Plant Cell Physiol. 48, 1775-1789.
Browse J. (2009). Jasmonate passes muster: a receptor and targets for the defense hormone. Ann. Rev. Plant Biol. 60, 183-205.
Chauvin A, Lenglet A, Wolfender JL, Farmer EE. (2016) Paired Hierarchical Organization of 13-Lipoxygenases in Arabidopsis. Plants 5(2), 16; doi: 10.3390/plants5020016
Nguyen CT, Kurenda A, Stolz S, Chételat A, Farmer EE. (2018) Identification of cell populations necessary for leaf-to-leaf electrical signaling in a wounded plant. Proc. Natl. Acad. Sci. USA 115, 10178-10183.
Park JH, Halitschke R, Kim HB, Baldwin IT, Feldmann KA, Feyerelsen R. (2002) A knock-out mutation in allene oxide synthase results in male sterility and defective wound signal transduction in Arabidopsis due to a block in jasmonic acid biosynthesis. Plant J. 318, 1-12.
Stintzi A, Browse J. (2000) The Arabidopsis male-sterile mutant, opr3, lacks the 12-oxophytodienoic acid reductase required for jasmonate synthesis. Proc. Natl. Acad. Sci. USA. 978, 10625-10630.
Zhang Y, Turner JG. (2008) Wound-induced endogenous jasmonates stunt plant growth by inhibiting mitosis. PLoS One 3, e3699