I Get by With a Little Help From a Friend
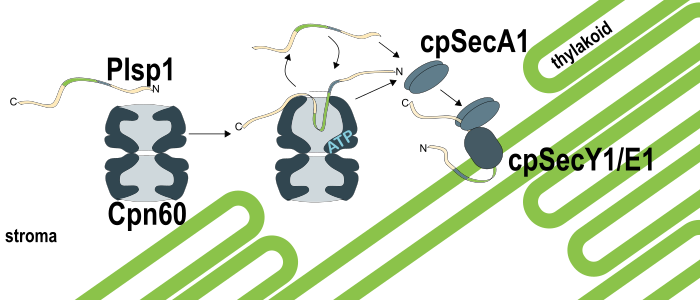
Klasek, Inoue, and Theg show how the chloroplast chaperonin Cpn60 binds Plastidic type I signal peptidase 1 in the chloroplast stroma and delivers it to the cpSecI translocon for targeting to the thylakoid membrane. Plant Cell https://doi.org/10.1105/tpc.20.00309
By Laura Klasek, University of California, Davis
Background: As a plant grows, its chloroplasts construct their internal thylakoid membranes, which begin harvesting light for photosynthesis. The thylakoid is protein-rich, and most thylakoid proteins are translated in the cytosol, including hydrophobic thylakoid membrane proteins. Once the chloroplast recognizes and imports these proteins, chaperonin proteins help them cross the aqueous stroma to reach the growing thylakoid membranes. However, how a certain chaperone helps a particular thylakoid membrane protein is mostly unknown. We are studying Plastidic type I signal peptidase 1 (Plsp1), a thylakoid membrane protein that must stay unfolded in the stroma until it reaches the thylakoid. We had previously observed Plsp1 in a large stromal protein complex that resembled a chaperone (Cpn60) known for helping proteins fold.
Questions: Does Plsp1 interact directly with Cpn60 and, if so, does Cpn60 help Plsp1 cross the stroma on its way to its final position in the thylakoid membrane?
Findings: We found that Cpn60 binds and releases Plsp1, which can then be delivered to the thylakoid via the cpSec1 translocon. When we isolated chloroplasts from pea seedlings and let them import Arabidopsis Plsp1 labeled with radioactive amino acids, we saw the radiolabeled signal first in the Cpn60-Plsp1 complex, then later in the thylakoid membrane. Cpn60 interacts with many stromal proteins, including the large subunit of Rubisco (RbcL), and when we added RbcL and ATP to Cpn60-Plsp1 complexes, Cpn60 released Plsp1 in favor of binding RbcL. If we added RbcL to Cpn60-Plsp1 complexes in the presence of thylakoids and the cpSec1 machinery that inserts Plsp1, Plsp1 reached the thylakoid successfully more efficiently.
Next steps: Open questions from our work include: How important is Cpn60 for Plsp1 targeting to the thylakoid?; Does Cpn60 assist other thylakoid proteins besides Plsp1? A better understanding of the role of Cpn60 in thylakoid development will enable more nuanced strategies to improve how plants use light.