The first structure of plant actin filaments
Ren et al. examine the structure of maize pollen actin.
Plant Cell https://doi.org/10.1105/tpc.18.00973
Zhanhong Ren (Ph.D. candidate), Key Laboratory of Cell Proliferation and Regulation Biology of Ministry of Education, Center for Biological Science and Technology, College of Life Sciences, Beijing Normal University, Zhuhai 519087, China
Haiyun Ren (professor), Key Laboratory of Cell Proliferation and Regulation Biology of Ministry of Education, Center for Biological Science and Technology, College of Life Sciences, Beijing Normal University, Zhuhai 519087, China
Background: The actin cytoskeleton in cells is like the steel reinforced concrete in a building. It plays vital roles in many fundamental processes including vesicle and organelle transportation, endo- and exocytosis, and cell division and growth. Actin exists as two states in vivo: globular actin (G-actin) and filamentous actin (F-actin), which are subject to a dynamic equilibrium of polymerization and depolymerization. In most instances, F-actin is the functional form of actin proteins. Thus, studying the structure of F-actin is of particular importance for understanding its functional mechanism. The biochemical activities and cellular functions are different between plant and animal actins. However, the structural basis accounting for these differences remains poorly understood, largely because none of the plant F-actin structures have been resolved.
Question: We wanted to determine the first structure of plant F-actin and discover its structural characteristics. Besides, we would like to measure the rupture forces of actin filaments.
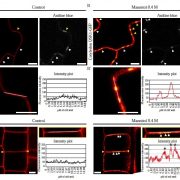
Findings: We report a 3.9 Å resolution structure of Zea mays (maize) pollen actin (ZMPA) filaments determined by cryo-electron microscopy and rupture forces of actin filaments measured by single-molecule magnetic tweezers. The structure shows a right-handed, double-stranded (two strands running parallel to each other) and staggered architecture. The DNase I-binding loop (D-loop) of the ZMPA filament structure bends further outward compared to that of the rabbit skeletal muscle actin (RSMA), adopting an open conformation similar to that of the jasplakinolide- or Beryllium fluoride (BeFx)-stabilized RSMA filament. Thus, plant actin filaments may be more stable than animal actin filaments. Single-molecule magnetic tweezers analysis revealed that the averaged rupture force of a single ZMPA filament is 37.8 pN (pico-Newton), which is 11.3 pN greater than that of a single RSMA filament. Our data provide evidence that plant actin filaments have a greater stability than animal actin filaments.
Next steps: Further work is needed to discover the direct connection between the structural characteristics of the plant actin cytoskeleton and physiological phenomena of plant cells, especially actins’ roles as tracks for long-distance vesicle and organelle transportation.
Zhanhong Ren, Yan Zhang, Yi Zhang, Yunqiu He, Pingzhou Du, Zhanxin Wang, Fei Sun, and Haiyun Ren. (2019). Cryo-EM Structure of Actin Filaments from Zea mays Pollen. Plant Cell; DOI: https://doi.org/10.1105/tpc.18.00973
Key words: Cryo-electron microscopy; D-loop conformation; F-actin stability; Single-molecule magnetic tweezers; Structure of plant actin filaments.