The CLP and PREP protease systems coordinate maturation and degradation of the chloroplast proteome in Arabidopsis thaliana (New Phytol.)
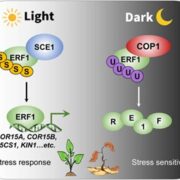
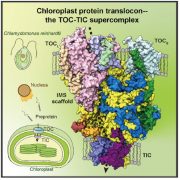
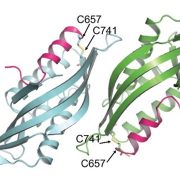
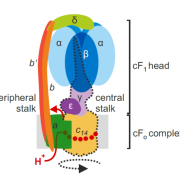
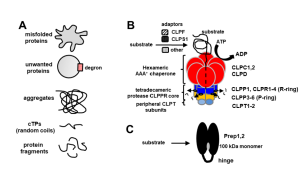 The ATP-dependent CLP chaperone-protease system is a crucial part of the peptidase network required to maintain proteostasis in the Arabidopsis chloroplast. Its substrates include misfolded and aggregated proteins of any size, and it is essential for plant growth and development. PREP1,2 peptidases are also part of this network but degrade small proteins and peptides such as cleaved chloroplast transit peptide (cTP) fragments and are targeted to both chloroplasts and mitochondria. Rowland et al. demonstrated the genetic interaction between these two systems by generating multiple combinations of higher order mutants and dissected the contributions of various components of these protease systems. The most severe growth defects were seen when both systems were defective, and the two PREP proteins are not redundant as previously thought. By using quantitative proteomics and N-terminal proteomics, the authors show that loss of CLP function causes protein folding stress in the cell and induces upregulation of a number of proteins such as an ATP-transporter, but the loss of PREP function does not impact this. Loss of both CLP and PREP1,2 function also inhibits the proper N-terminal processing of chloroplast-imported proteins by other peptidases. (Summary by Jiawen Chen @jiaaawen) New Phytol. 10.1111/nph.18426.
The ATP-dependent CLP chaperone-protease system is a crucial part of the peptidase network required to maintain proteostasis in the Arabidopsis chloroplast. Its substrates include misfolded and aggregated proteins of any size, and it is essential for plant growth and development. PREP1,2 peptidases are also part of this network but degrade small proteins and peptides such as cleaved chloroplast transit peptide (cTP) fragments and are targeted to both chloroplasts and mitochondria. Rowland et al. demonstrated the genetic interaction between these two systems by generating multiple combinations of higher order mutants and dissected the contributions of various components of these protease systems. The most severe growth defects were seen when both systems were defective, and the two PREP proteins are not redundant as previously thought. By using quantitative proteomics and N-terminal proteomics, the authors show that loss of CLP function causes protein folding stress in the cell and induces upregulation of a number of proteins such as an ATP-transporter, but the loss of PREP function does not impact this. Loss of both CLP and PREP1,2 function also inhibits the proper N-terminal processing of chloroplast-imported proteins by other peptidases. (Summary by Jiawen Chen @jiaaawen) New Phytol. 10.1111/nph.18426.