Review. Chloroplast ATP synthase: From structure to engineering
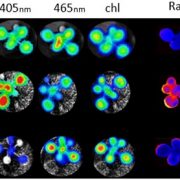
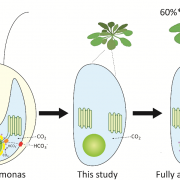
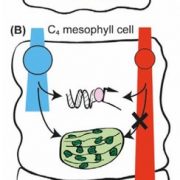
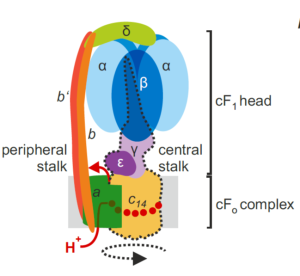 I remember how amazed I was the first time I saw an animation of ATP synthase doing its job. This fantastic engine is largely conserved across the domains of life, with some variation as highlighted in this review of the chloroplast ATP synthase by Rühle et al. The plastid form is a rotary form, written as cF1F0. The cF0 subunit is embedded in the thylakoid membrane, whereas the cF1 subunit protrudes into the stroma. Protons move through it (driven by proton-motive force generated by photosynthesis) from within the lumen across the membrane into the stroma, and their movement is coupled with the phosphorylation of ADP to ATP. The review is divided into three parts: structure, assembly, and engineering, and I particularly enjoyed the discussion of how the chloroplast enzyme is regulated by redox, pH, and in turn regulates the light reactions including the balance between cyclic and linear electron flow and PSII and PSI activities. Because of the intricate connection between protons, electrons, light reactions and ATP, efforts to make the ATPase more efficient are challenging but intriguing. However, given the many efforts to improve other components of photosynthetic machinery, such as the introduction of carbon-concentrating mechanisms, understanding how such efforts might need to also be supported through engineering the chloroplast ATPase is critical. Note that this review is part of an upcoming Plant Cell Focus Issue on Photosynthesis. (Summary by Mary Williams @PlantTeaching) Plant Cell 10.1093/plcell/koae081
I remember how amazed I was the first time I saw an animation of ATP synthase doing its job. This fantastic engine is largely conserved across the domains of life, with some variation as highlighted in this review of the chloroplast ATP synthase by Rühle et al. The plastid form is a rotary form, written as cF1F0. The cF0 subunit is embedded in the thylakoid membrane, whereas the cF1 subunit protrudes into the stroma. Protons move through it (driven by proton-motive force generated by photosynthesis) from within the lumen across the membrane into the stroma, and their movement is coupled with the phosphorylation of ADP to ATP. The review is divided into three parts: structure, assembly, and engineering, and I particularly enjoyed the discussion of how the chloroplast enzyme is regulated by redox, pH, and in turn regulates the light reactions including the balance between cyclic and linear electron flow and PSII and PSI activities. Because of the intricate connection between protons, electrons, light reactions and ATP, efforts to make the ATPase more efficient are challenging but intriguing. However, given the many efforts to improve other components of photosynthetic machinery, such as the introduction of carbon-concentrating mechanisms, understanding how such efforts might need to also be supported through engineering the chloroplast ATPase is critical. Note that this review is part of an upcoming Plant Cell Focus Issue on Photosynthesis. (Summary by Mary Williams @PlantTeaching) Plant Cell 10.1093/plcell/koae081