Altering the location of starch granules by relocalizing a starch granule initiation protein
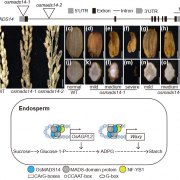
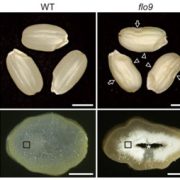
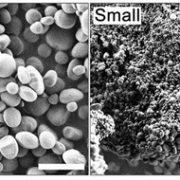
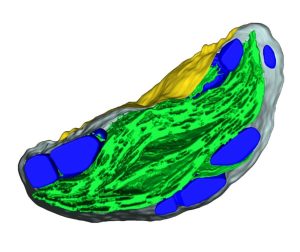 Starch granules are synthesized in leaf chloroplasts during the day and degraded at night to provide carbon. MFP1 (MAR-BINDING FILAMENT-LIKE PROTEIN 1) is known to have a role in starch granule initiation, but its mechanistic role has been unknown. Sharma et al. hypothesized MFP1’s thylakoid localization signals are important for its role; to test this they swapped the transit peptide, thylakoid signal peptide, and transmembrane domains from MFP1 for the N-terminal region of the chloroplast inner envelope protein Tic40 (TRANSLOCON AT THE INNER ENVELOPE MEMBRANE OF CHLOROPLASTS 40); they call this new protein Tic40NMFP1. When Tic40NMFP1 is expressed under a ubiquitin promoter in mfp1 Arabidopsis plants, MFP1 was not found at the thylakoid membranes but instead localized to the chloroplast periphery. Interestingly, they saw relocalization of other key starch granule initiation proteins, including SS4 (STARCH SYTHASE 4) and PTST2 (PROTEIN TARGETING TO STARCH 2), to the same location. Analysis of chloroplast ultrastructure by focused ion beam-scanning electron microscopy revealed that starch granules were also forming at the periphery of the chloroplast, rather than near the thylakoids, and these were smaller and rounder than those in wild type plants. Hence MFP1 is important for localizing granule initiation proteins and ensuring starch granules form near thylakoid membranes. (Summary by Rose McNelly @Rose_McN) Proc. Natl. Acad. Sci. USA 10.1073/pnas.2309666121
Starch granules are synthesized in leaf chloroplasts during the day and degraded at night to provide carbon. MFP1 (MAR-BINDING FILAMENT-LIKE PROTEIN 1) is known to have a role in starch granule initiation, but its mechanistic role has been unknown. Sharma et al. hypothesized MFP1’s thylakoid localization signals are important for its role; to test this they swapped the transit peptide, thylakoid signal peptide, and transmembrane domains from MFP1 for the N-terminal region of the chloroplast inner envelope protein Tic40 (TRANSLOCON AT THE INNER ENVELOPE MEMBRANE OF CHLOROPLASTS 40); they call this new protein Tic40NMFP1. When Tic40NMFP1 is expressed under a ubiquitin promoter in mfp1 Arabidopsis plants, MFP1 was not found at the thylakoid membranes but instead localized to the chloroplast periphery. Interestingly, they saw relocalization of other key starch granule initiation proteins, including SS4 (STARCH SYTHASE 4) and PTST2 (PROTEIN TARGETING TO STARCH 2), to the same location. Analysis of chloroplast ultrastructure by focused ion beam-scanning electron microscopy revealed that starch granules were also forming at the periphery of the chloroplast, rather than near the thylakoids, and these were smaller and rounder than those in wild type plants. Hence MFP1 is important for localizing granule initiation proteins and ensuring starch granules form near thylakoid membranes. (Summary by Rose McNelly @Rose_McN) Proc. Natl. Acad. Sci. USA 10.1073/pnas.2309666121