Stop the Clock: Optimized Carbon Fixation and Circadian Rhythm in a CAM Plant
The energetically costly tendency of the carbon fixing enzyme RuBisCO to, every now and then, fix oxygen instead of carbon dioxide has led to the evolution of various carbon concentrating mechanisms in plants and algae. One such mechanism, Crassulacean acid metabolism (CAM), involves primary CO2 fixation in the dark and secondary fixation by RuBisCO in the light. The day/night separation of these fixation events allows stomatal closure during the day, granting CAM species increased water-use efficiency compared to C3 or C4 plants, a trait that may become more advantageous in the context of global rises in temperature and drought intensity. However, this temporal, as opposed to spatial, separation comes with certain caveats: the various CAM processes must be under tight circadian control in order to prevent futile cycling of metabolites. In recent work by Boxall et al. (2017), an RNAi approach was utilized to reveal clues about the nature of this control in the model obligate CAM species Kalanchoë fedtschenkoi.
Despite CO2 uptake and fixation occurring almost entirely at night in K. fedtschenkoi, both the abundance and the specific activity of phosphoenolpyruvate carboxylase (PPC), the enzyme responsible for primary CO2 fixation, remains fairly stable across light/dark cycles. The circadian regulation of this process has therefore been linked to PPC kinase (PPCK), which shows both peak transcript accumulation and enzyme activity in the mid-to-late night. PPCK phosphorylates PPC and in doing so reduces the allosteric inhibition of PPC by malate (Carter et al., 1991) the downstream product of primary CO2 fixation that accumulates in CAM plants.
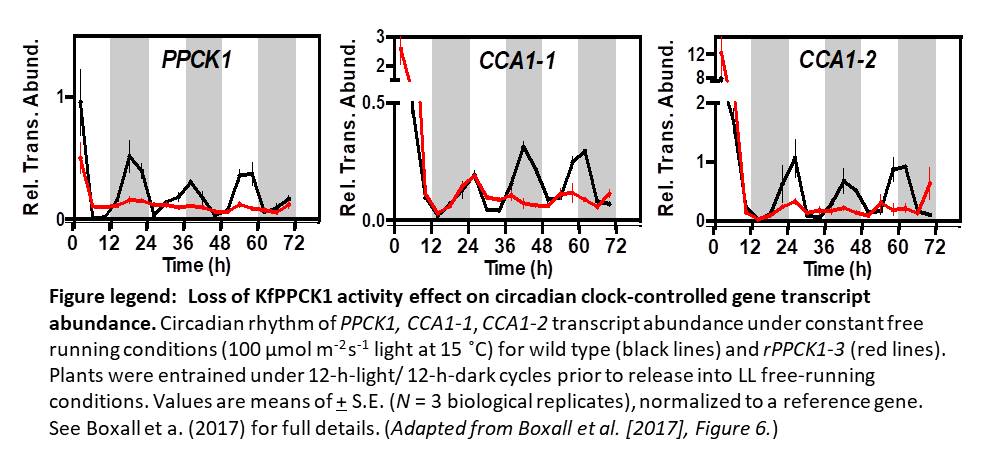
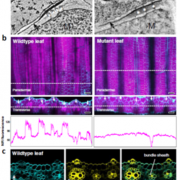
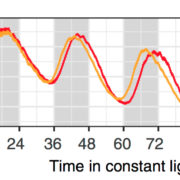
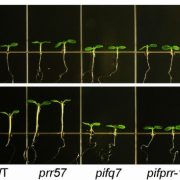
To investigate the role of PPCK as a core circadian regulator of CO2 fixation, Boxall et al. isolated and characterized K. fedtschenkoi PPCK RNAi lines. Correlating to reductions in the PPCK transcript, which were particularly diminished relative to wild-type peaks during the dark periods, nighttime phosphorylation of PPC was relaxed prematurely in a mild line (rPPCK1-1), and barely detectable in the strong line (rPPCK1-3). Linked to this was a PPCK dosage-dependent increase in the inhibition of PPC by malate, a decrease in the length of dark period CO2 fixation, a decrease in the levels of malate accumulation, and a decrease in the nighttime turnover of starch.
Together, these results suggest that PPCK phosphorylation of PPC is necessary to optimize nighttime CO2 fixation in CAM plants. And while it follows that simple circadian control of PPCK accumulation may be used to regulate this fixation, Boxall et al., noted a more complex relationship between the kinase and components of the central oscillator. In addition to a more rapid dampening of CO2 fixation periodicity under free running conditions, rPPCK1-3 also showed altered accumulation and periodicity of core circadian clock-related transcripts, such as TOC1 and CCA1. This finding disrupts the notion that PPCK is merely a circadian controlled output, suggesting that PPCK rather acts in a feedback loop that maintains global plant rhythmicity. While future work may help elucidate the exact mechanism of this feedback, the authors suggest that metabolic changes arising from PPCK activity, perhaps the accumulation of sucrose, may be involved.
This work has particular implications for ongoing efforts to engineer CAM processes into C3 crops, by demonstrating that peak CAM productivity can only occur in the context of the circadian clock.
References
Carter, P.J., Nimmo, H.G., Fewson, C.A., and Wilkins, M.B. (1991). Circadian-Rhythms in the Activity of a Plant Protein-Kinase. Embo J 10: 2063-2068.
Boxall, S.F., Dever, L. V., Kneřová, J., Gould, P.D., Hartwell, J. (2017). Phosphorylation of Phosphoenolpyruvate Caboxylase is Essential for Maximal and Sustained Dark CO2 Fixation and Core Circadian Clock Operation in the Obligate Crassulacean Acid Metabolism Species Kalanchoë fedtschenkoi. Plant Cell . Published September 8, 2017 doi.org/10.1105/tpc.17.00301