Persulfidation of ATG4 negatively regulates autophagy
Ana M. Laureano-Marín, Ángeles Aroca, et al. explore the role of persulfidation in the regulation of autophagy. Plant Cell https://doi.org/10.1105/tpc.20.00766
by Cecilia Gotor, Ana M. Laureano-Marín, Ángeles Aroca
Instituto de Bioquímica Vegetal y Fotosíntesis, Consejo Superior de Investigaciones Científicas and Universidad de Sevilla, Seville, Spain
Background: Hydrogen sulfide (H2S) is a poisonous substance hazardous to life and the environment, but it is also present in biological tissues. Intense investigation showed that H2S functions as an important regulator of essential processes in animals and plants. For example, our previous research on the plant Arabidopsis demonstrated that H2S regulates autophagy. In autophagy (which is conserved in plants and animals), cell contents are digested for recycling. The materials to be digested are sequestered in a double membrane-bound structures called autophagosomes and the proteins involved in the main molecular machinery are referred to as autophagy-related (ATG). Autophagy is involved in plant development, immune responses, and adaptation to adverse environmental conditions.
Question: We wanted to know the mechanism of action by which H2S regulates autophagy. Previous findings showed that H2S is not a reductant in autophagy. We want to determine if the mechanism is persulfidation, a posttranslational protein modification of the thiol group of cysteines to form a persulfide group, and to identify the target ATG proteins.
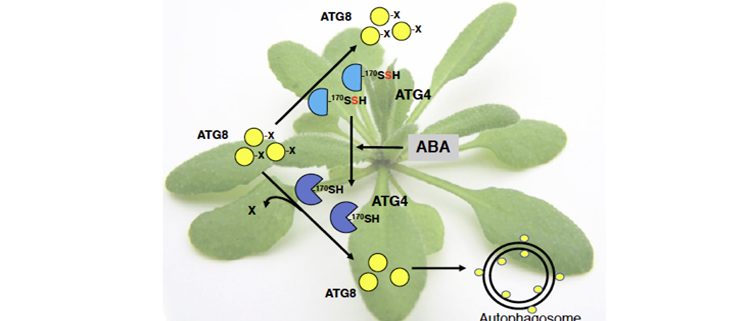
Findings: Using proteomic analysis of Arabidopsis leaves treated with the hormone abscisic acid (ABA), we found that persulfidation of the cysteine protease ATG4 controls autophagy. When the persulfidation level of ATG4 is reduced after a short ABA treatment the autophagy progresses. This ATG4 protease undergoes specific persulfidation of the Cys170 residue that is a part of the catalytic site. ATG4 catalyzes the processing of newly synthetized ATG8, which is essential for the synthesis of autophagosomes. This activity was measured in a heterologous assay using Chlamydomonas ATG8 as substrate and we demonstrated that H2S significantly and reversibly inactivates the proteolytic activity of ATG4. Under autophagy-inducing conditions such as nitrogen starvation and osmotic stress, we detected a significant increase in the overall ATG4 proteolytic activity that can be inhibited by sulfide.
Next steps: Our study demonstrated that negative regulation of autophagy by H2S is mediated by persulfidation of ATG4. Probably this is not the only protein and additional targets remain to be identified. We will also investigate the role of H2S in the regulation of selective autophagy and the interplay between H2S and other regulators.
Ana M. Laureano-Marín, Ángeles Aroca, M. Esther Pérez-Pérez, Inmaculada Yruela, Ana Jurado-Flores, Inmaculada Moreno, José L. Crespo, Luis C. Romero, Cecilia Gotor (2020) Abscisic Acid-Triggered Persulfidation of the Cysteine Protease ATG4 Mediates Regulation of Autophagy by Sulfide. https://doi.org/10.1105/tpc.20.00766