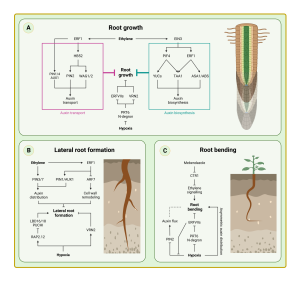
CYCLOIDEA-like genes control multiple floral traits
Yang, Wang, Liu, et al. reveal that CYCLOIDEA-like genes control the genetic correlation of floral symmetry, floral orientation, and nectar guide patterns.
https://doi.org/10.1093/plcell/koad115
By Xia Yang1,2 and Yin-Zheng Wang1,2,3
Institutions:
1 State Key Laboratory of Systematic and Evolutionary Botany, Institute of Botany,
Chinese Academy of Sciences, Xiangshan, Beijing 100093, China
2 China National Botanical Garden, Beijing 100093, China
3 College of Life Sciences, University of Chinese Academy of Sciences, Beijing 100049,
China
Background: Three types of floral symmetry can be distinguished based on the number of symmetry planes: polysymmetry (with several symmetry planes), monosymmetry (with only one symmetry plane), and asymmetry. Early angiosperms have polysymmetric floral organs, while monosymmetry originated many times from polysymmetry, and several large clades produce predominantly or entirely monosymmetric flowers. In addition to having differential morphologies and sizes in the second and third whorls of floral organs, monosymmetric flowers usually possess horizontal orientation and asymmetric nectar guides. CYCLOIDEA (CYC)-like TCP transcription factors control floral monosymmetry in many species, but little is known about how horizontal orientation and asymmetric nectar guides are achieved.
Question: Are floral symmetry, floral orientation, and nectar guide patterning correlated traits, and are they determined by the same set of master regulators, such as CYC-like genes?
Findings: We selected Chirita pumila (Gesneriaceae) as a model system to address this issue. Plants overexpressing CpCYC1 and CpCYC2 generated dorsalized flowers, with a change in floral orientation from horizontal to upward and the loss of yellow nectar guides in the ventral corolla tube. By contrast, the cyc1 cyc2 double mutant produced ventralized flowers with upward orientation and uniform yellow nectar guides. Therefore, CpCYC1 and CpCYC2 not only determine floral symmetry, but they also regulate floral orientation and nectar guide patterning. CpCYC1 positively regulates itself and down-regulates CpCYC2, while CpCYC2 up-regulates CpCYC1. We also identified the flavonoid biosynthesis-related gene CpF3’5’H, which regulates yellow nectar guide formation. CpCYC1 and CpCYC2 repress yellow pigment formation in nectar guides out the ventral region of the flower, likely by directly repressing CpF3’5’H.
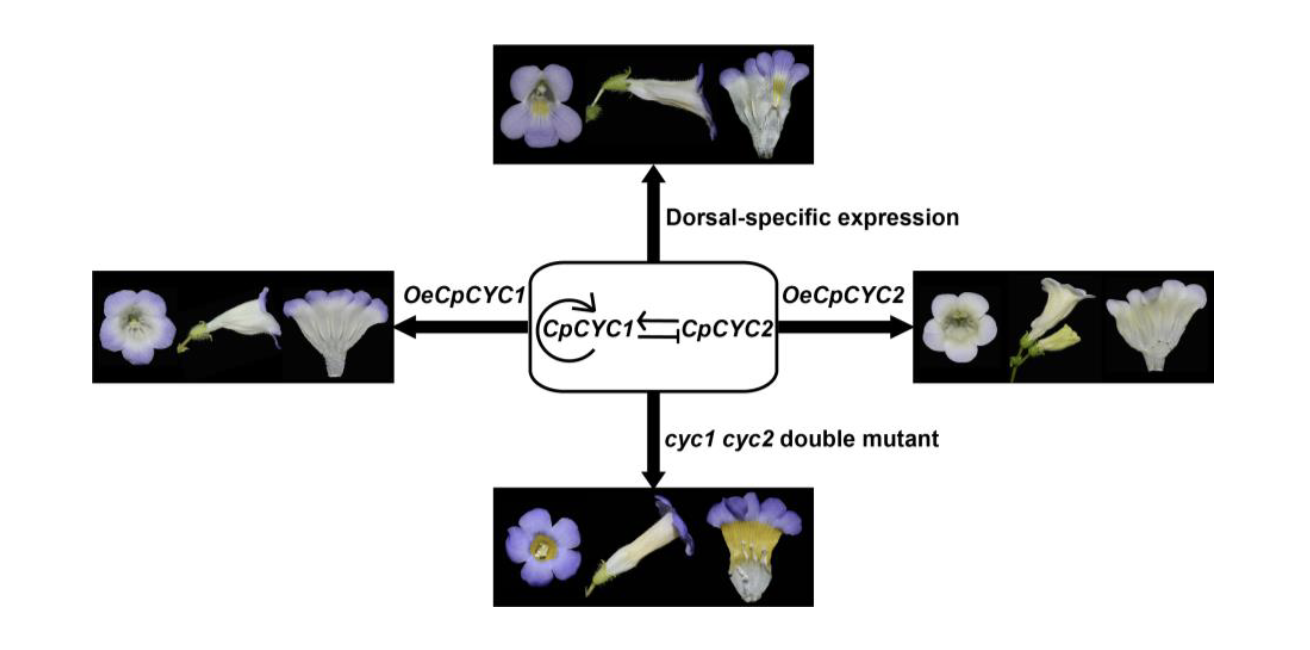 Next steps: Further studies are needed to elucidate how CpCYC1 and CpCYC2 control these distinct floral traits by regulating different target genes or interacting with different co-factors.
Next steps: Further studies are needed to elucidate how CpCYC1 and CpCYC2 control these distinct floral traits by regulating different target genes or interacting with different co-factors.
Xia Yang, Yang Wang, Tian-Xia Liu, Qi Liu, Jing Liu, Tian-Feng Lü, Rui-Xue Yang, Feng-Xian Guo and Yin-Zheng Wang. (2023). CYCLOIDEA-like genes control floral symmetry, floral orientation, and nectar guide patterning. https://doi.org/10.1093/plcell/koad115


 Next steps:
Next steps:
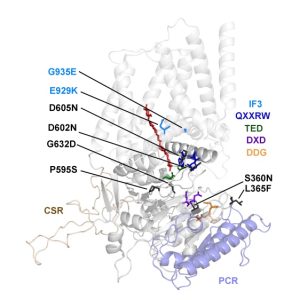 Findings: We created and tested 18 single amino acid replacement mutations in the CESA6 catalytic domain. Using quantitative live-cell imaging of the dynamics of CESA6 labeled with the yellow fluorescent protein (YFP-CESA6), we found that multiple mutations in the DXD, TED and QXXRW motifs, but not the DDG motif, caused reduced motility of PM-localized CSCs, indicative of reduced cellulose biosynthesis activity. Additionally, various mutations affected Golgi-to-PM trafficking of the CSC. Using principal component analysis of nine biochemical and cellular parameters, the 18 mutations separated into two major groups. Group I mutations caused CSC trafficking defects whereas Group II mutations, especially in the QXXRW motif, affected CESA protein folding and/or CSC rosette formation in the ER or Golgi with consequences for post-Golgi trafficking.
Findings: We created and tested 18 single amino acid replacement mutations in the CESA6 catalytic domain. Using quantitative live-cell imaging of the dynamics of CESA6 labeled with the yellow fluorescent protein (YFP-CESA6), we found that multiple mutations in the DXD, TED and QXXRW motifs, but not the DDG motif, caused reduced motility of PM-localized CSCs, indicative of reduced cellulose biosynthesis activity. Additionally, various mutations affected Golgi-to-PM trafficking of the CSC. Using principal component analysis of nine biochemical and cellular parameters, the 18 mutations separated into two major groups. Group I mutations caused CSC trafficking defects whereas Group II mutations, especially in the QXXRW motif, affected CESA protein folding and/or CSC rosette formation in the ER or Golgi with consequences for post-Golgi trafficking.