Turnover of Complex I is regulated by FTSH3 that recognises the PSST subunit
Ghifari et al. explore the mechanisms of Complex I disassembly and turnover.
https://doi.org/10.1093/plcell/koad128
Abi S. Ghifari and Monika W Murcha
School of Molecular Sciences, The University of Western Australia
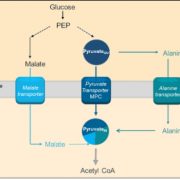
Background: Oxidative phosphorylation (OXPHOS) is the central process of aerobic respiration in plant mitochondria. Complex I, the first entry point and largest complex of the OXPHOS pathway, begins the OXPHOS process by oxidizing the high-energy intermediate NADH and transferring electrons to the mobile electron carrier ubiquinone. High redox activity and constant exposure to reactive oxygen species render Complex I subunits prone to oxidative damage, resulting in a high turnover rate. Despite recent advances in the structural determination of plant Complex I, how Complex I degradation and turnover is regulated remains enigmatic.
Question: Which factors determine Complex I turnover, and how is this process mechanistically regulated?
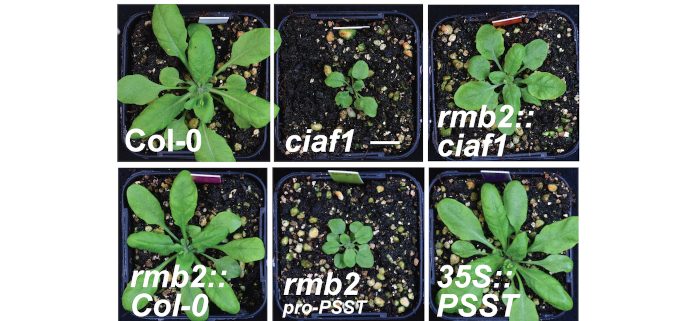
Findings: Using two independent Arabidopsis thaliana EMS mutants generated in a Complex I defective background, we show that FTSH3, a mitochondrial matrix-facing inner membrane-bound protease, facilitates the unfolding of Complex I matrix arm subunits for degradation and turnover. For this function, FTSH3 interacts directly with PSST, a Complex I matrix arm domain subunit. This interaction is mediated by the ATPase domain of FTSH3 and the N-terminal domain of PSST. Mutations in these domains prevent the interaction between these two factors, slowing the turnover rate of matrix arm subunits, resulting in enhanced Complex I subunit abundance and activity.
Next steps: We plan to assess the specificity of FTSH3 in regulating the turnover of other OXPHOS complexes, as well as its modulation of the activities of other mitochondrial proteases. By identifying the role of FTSH3 with regards to other OXPHOS subunits and proteases, we can provide a more comprehensive view of how of mitochondrial OXPHOS protein turnover is regulated.
Reference:
Abi S. Ghifari, Aneta Ivanova, Oliver Berkowitz, James Whelan, Monika W. Murcha. (2023). FTSH PROTEASE 3 facilitates complex I degradation through a direct interaction with the complex I subunit PSST https://doi.org/10.1093/plcell/koad128