The catalytic domain of cellulose synthase: More than just cellulose biosynthesis
Huang et al. demonstrate that the conserved catalytic domain of cellulose synthase 6 (CESA6) is involved in trafficking, protein dynamics, and complex formation. The Plant Cell (2023)
https://doi.org/10.1093/plcell/koad110
Lei Huang,1,2 Weiwei Zhang,1,3 Xiaohui Li,1,2 and Christopher J. Staiger1,2,3
1Department of Botany and Plant Pathology, Purdue University, West Lafayette, IN, 47907, USA
2Center for Plant Biology, College of Agriculture, Purdue University, West Lafayette, IN, 47907, USA
3Department of Biological Sciences, Purdue University, West Lafayette, IN, 47907, USA
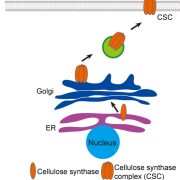
Background: The cell wall plays a vital role in the survival and growth of plants, and provides abundant renewable biopolymers, like cellulose, that have significant economic importance. Cellulose biosynthesis occurs at the cell surface through a plasma membrane (PM)-localized multimeric protein complex named the cellulose synthase (CESA) complex (CSC). CESAs are synthesized in the endoplasmic reticulum, transported to the Golgi for CSC complex assembly, and delivered to the PM through secretory vesicles. Recently, we identified a small molecule inhibitor, Endosidin 20, that targets the catalytic domain of CESA6 and apparently affects CSC trafficking to the PM.
Question: The large catalytic domain of plant CESA contains several key motifs that are also present in bacterial CESAs. How do these conserved motifs and the catalytic domain of plant CESAs contribute to CESA protein dynamics, complex formation and/or trafficking?
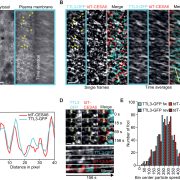
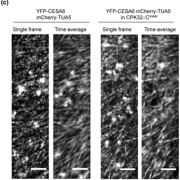
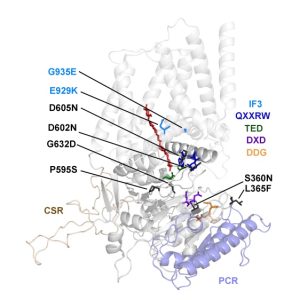 Findings: We created and tested 18 single amino acid replacement mutations in the CESA6 catalytic domain. Using quantitative live-cell imaging of the dynamics of CESA6 labeled with the yellow fluorescent protein (YFP-CESA6), we found that multiple mutations in the DXD, TED and QXXRW motifs, but not the DDG motif, caused reduced motility of PM-localized CSCs, indicative of reduced cellulose biosynthesis activity. Additionally, various mutations affected Golgi-to-PM trafficking of the CSC. Using principal component analysis of nine biochemical and cellular parameters, the 18 mutations separated into two major groups. Group I mutations caused CSC trafficking defects whereas Group II mutations, especially in the QXXRW motif, affected CESA protein folding and/or CSC rosette formation in the ER or Golgi with consequences for post-Golgi trafficking.
Findings: We created and tested 18 single amino acid replacement mutations in the CESA6 catalytic domain. Using quantitative live-cell imaging of the dynamics of CESA6 labeled with the yellow fluorescent protein (YFP-CESA6), we found that multiple mutations in the DXD, TED and QXXRW motifs, but not the DDG motif, caused reduced motility of PM-localized CSCs, indicative of reduced cellulose biosynthesis activity. Additionally, various mutations affected Golgi-to-PM trafficking of the CSC. Using principal component analysis of nine biochemical and cellular parameters, the 18 mutations separated into two major groups. Group I mutations caused CSC trafficking defects whereas Group II mutations, especially in the QXXRW motif, affected CESA protein folding and/or CSC rosette formation in the ER or Golgi with consequences for post-Golgi trafficking.
Next steps: CESA-associated proteins participate in plant tolerance to salt and drought stresses, CSC exocytosis, etc. through interactions with the catalytic domain of CESA. It will be interesting to test how our collection of point mutations in CESA6 affect plant response to adverse stresses and/or modulate interactions with CESA-associated proteins.
Reference:
Huang L., Zhang W., Li X., Staiger C.J., Zhang C. (2023). Point mutations in the catalytic domain disrupt cellulose synthase (CESA6) vesicle trafficking and protein dynamics. https://doi.org/10.1093/plcell/koad110